|
Aerogel
Aerogels are a class of manufacturing, synthetic porous ultralight material derived from a gel, in which the liquid component for the gel has been replaced with a gas, without significant collapse of the gel structure. The result is a solid with extremely low density and extremely low thermal conductivity. Aerogels can be made from a variety of chemical compounds. Silica aerogels feel like fragile expanded polystyrene, styrofoam to the touch, while some polymer-based aerogels feel like rigid foams. Aerogels are produced by extracting the liquid component of a gel through supercritical drying or freeze-drying. This allows the liquid to be slowly dried off without causing the solid matrix in the gel to collapse from capillary action, as would happen with conventional evaporation. The first aerogels were produced from silica gels. Kistler's later work involved aerogels based on alumina, Chromium(III) oxide, chromia, and tin dioxide. Carbon aerogels were first developed in the late ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultralight Material
Ultralight materials are solids with a density of less than 10 mg/cm3, including silica aerogels, carbon nanotube aerogels, aerographite, metallic foams, polymeric foams, and metallic microlattices. The density of air is about 1.275 mg/cm3, which means that the air in the pores contributes significantly to the density of these materials in atmospheric conditions. They can be classified by production method as aerogels, stochastic foams, and structured cellular materials. Properties Ultralight materials are solids with a density of less than 10 mg/cm3. Ultralight material is defined by its cellular arrangement and its stiffness and strength that make up its solid constituent. They include silica aerogels, carbon nanotube aerogels, aero graphite, metallic foams, polymeric foams, and metallic micro lattices. Ultralight materials are produced to have the strength of bulk-scaled properties at a micro-size. Also, they are designed to not compress even under extreme p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samuel Stephens Kistler
Samuel Stephens Kistler (March 26, 1900 November 6, 1975) was an American scientist and chemical engineer, best known as the inventor of aerogels, one of the lightest known solid materials. Biography Kistler, the son of a shopkeeper, was born in the small town of Cedarville in the far northeastern corner of California. The family moved to the larger Santa Rosa when Kistler was 12, where he first became interested in chemistry. When he entered the College of the Pacific in 1917, however, his plan was to learn to play the cello, then pursue a degree in agriculture. Instead, he ended up taking every science course available, and after three years he moved to Stanford University and obtained a B.A. in chemistry, followed by a chemical engineering degree. He never did learn to play the cello. After a brief spell working for the Standard Oil Company of California, he returned to academia, teaching chemistry at the College of the Pacific until 1931, when he transferred to the Universi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silica Gel
Silica gel is an amorphous and porosity, porous form of silicon dioxide (silica), consisting of an irregular three-dimensional framework of alternating silicon and oxygen atoms with nanometer-scale voids and pores. The voids may contain water or some other liquids, or may be filled by gas or vacuum. In the last case, the material is properly called silica Gel#Xerogels, xerogel. Silica xerogel with an average pore size of 2.4 nanometers has a strong affinity for water molecules and is widely used as a desiccant. It is hard and translucence, translucent, but considerably softer than massive silica glass or quartz, and remains hard when saturated with water. Silica xerogel is usually commercialized as coarse granules or beads, a few millimeters in diameter. Some grains may contain small amounts of indicator substance that changes color when they have absorbed some water. Small paper envelopes containing silica xerogel pellets, usually with a "do not eat" warning, are often i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Density
Density (volumetric mass density or specific mass) is the ratio of a substance's mass to its volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' (or ''d'') can also be used: \rho = \frac, where ''ρ'' is the density, ''m'' is the mass, and ''V'' is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate this quantity is more specifically called specific weight. For a pure substance, the density is equal to its mass concentration. Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium is the densest known element at standard conditions for temperature and pressure. To simplify comparisons of density across different systems of units, it is sometimes replaced by the dimensionless quantity "relative den ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Friability
In materials science, friability ( ), the condition of being friable, describes the tendency of a solid substance to break into smaller pieces under stress or contact, especially by rubbing. The opposite of friable is indurate. Substances that are designated hazardous, such as asbestos or crystalline silica, are often said to be friable if small particles are easily dislodged and become airborne, and hence respirable (able to enter human lungs), thereby posing a health hazard. Tougher substances, such as concrete, may also be mechanically ground down and reduced to finely divided mineral dust. However, such substances are not generally considered friable because of the degree of difficulty involved in breaking the substance's chemical bonds through mechanical means. Some substances, such as polyurethane foams, show an increase in friability with exposure to ultraviolet radiation, as in sunlight. Friable is sometimes used metaphorically to describe "brittle" personalities wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid
Liquid is a state of matter with a definite volume but no fixed shape. Liquids adapt to the shape of their container and are nearly incompressible, maintaining their volume even under pressure. The density of a liquid is usually close to that of a solid, and much higher than that of a gas. Therefore, liquid and solid are classified as condensed matter. Meanwhile, since both liquids and gases can flow, they are categorized as fluids. A liquid is composed of atoms or molecules held together by intermolecular bonds of intermediate strength. These forces allow the particles to move around one another while remaining closely packed. In contrast, solids have particles that are tightly bound by strong intermolecular forces, limiting their movement to small vibrations in fixed positions. Gases, on the other hand, consist of widely spaced, freely moving particles with only weak intermolecular forces. As temperature increases, the molecules in a liquid vibrate more intensely, causi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tin Dioxide
Tin(IV) oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names, this oxide of tin is an important material in tin chemistry. It is a colourless, diamagnetic, amphoteric solid. Structure Tin(IV) oxide crystallises with the rutile structure. As such the tin atoms are six coordinate and the oxygen atoms three coordinate. SnO2 is usually regarded as an oxygen-deficient n-type semiconductor. Hydrous forms of SnO2 have been described as stannic acid. Such materials appear to be hydrated particles of SnO2 where the composition reflects the particle size. Preparation Tin(IV) oxide occurs naturally. Synthetic tin(IV) oxide is produced by burning tin metal in air. Annual production is in the range of 10 kilotons. SnO2 is reduced industrially to the metal with carbon in a reverberatory furnace at 1200–1300 °C. Reactions The reaction from tin(IV) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porosity
Porosity or void fraction is a measure of the void (i.e. "empty") spaces in a material, and is a fraction of the volume of voids over the total volume, between 0 and 1, or as a percentage between 0% and 100%. Strictly speaking, some tests measure the "accessible void", the total amount of void space accessible from the surface (cf. closed-cell foam). There are many ways to test porosity in a substance or part, such as industrial CT scanning. The term porosity is used in multiple fields including pharmaceutics, ceramics, metallurgy, materials, manufacturing, petrophysics, hydrology, earth sciences, soil mechanics, rock mechanics, and engineering. Void fraction in two-phase flow In gas-liquid two-phase flow, the void fraction is defined as the fraction of the flow-channel volume that is occupied by the gas phase or, alternatively, as the fraction of the cross-sectional area of the channel that is occupied by the gas phase. Void fraction usually varies from location to l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanometre
330px, Different lengths as in respect to the Molecule">molecular scale. The nanometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: nm), or nanometer (American spelling), is a unit of length in the International System of Units (SI), equal to one billionth ( short scale) or one thousand million (long scale) of a meter (0.000000001 m) and to 1000 picometres. One nanometre can be expressed in scientific notation as 1 × 10−9 m and as m. History The nanometre was formerly known as the "''millimicrometre''" – or, more commonly, the "''millimicron''" for short – since it is of a micrometer. It was often denoted by the symbol ''mμ'' or, more rarely, as ''μμ'' (however, ''μμ'' should refer to a ''millionth'' of a micron). Etymology The name combines the SI prefix '' nano-'' (from the Ancient Greek , ', "dwarf") with the parent unit name ''metre'' (from Greek , ', "unit of measurement"). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
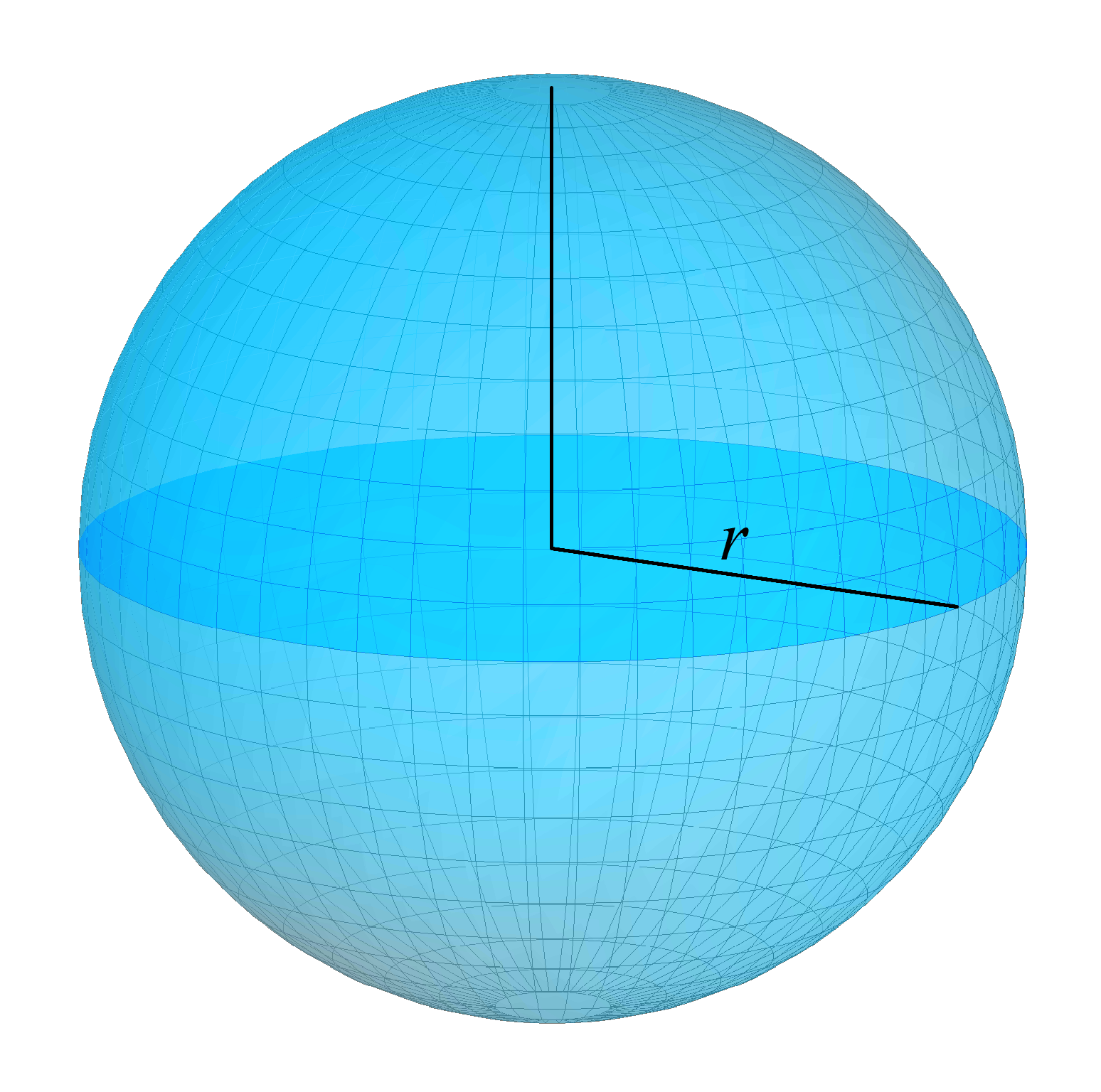
Spherical
A sphere (from Ancient Greek, Greek , ) is a surface (mathematics), surface analogous to the circle, a curve. In solid geometry, a sphere is the Locus (mathematics), set of points that are all at the same distance from a given point in three-dimensional space.. That given point is the center (geometry), ''center'' of the sphere, and the distance is the sphere's ''radius''. The earliest known mentions of spheres appear in the work of the Greek mathematics, ancient Greek mathematicians. The sphere is a fundamental surface in many fields of mathematics. Spheres and nearly-spherical shapes also appear in nature and industry. Bubble (physics), Bubbles such as soap bubbles take a spherical shape in equilibrium. The Earth is spherical Earth, often approximated as a sphere in geography, and the celestial sphere is an important concept in astronomy. Manufactured items including pressure vessels and most curved mirrors and lenses are based on spheres. Spheres rolling, roll smoothly in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
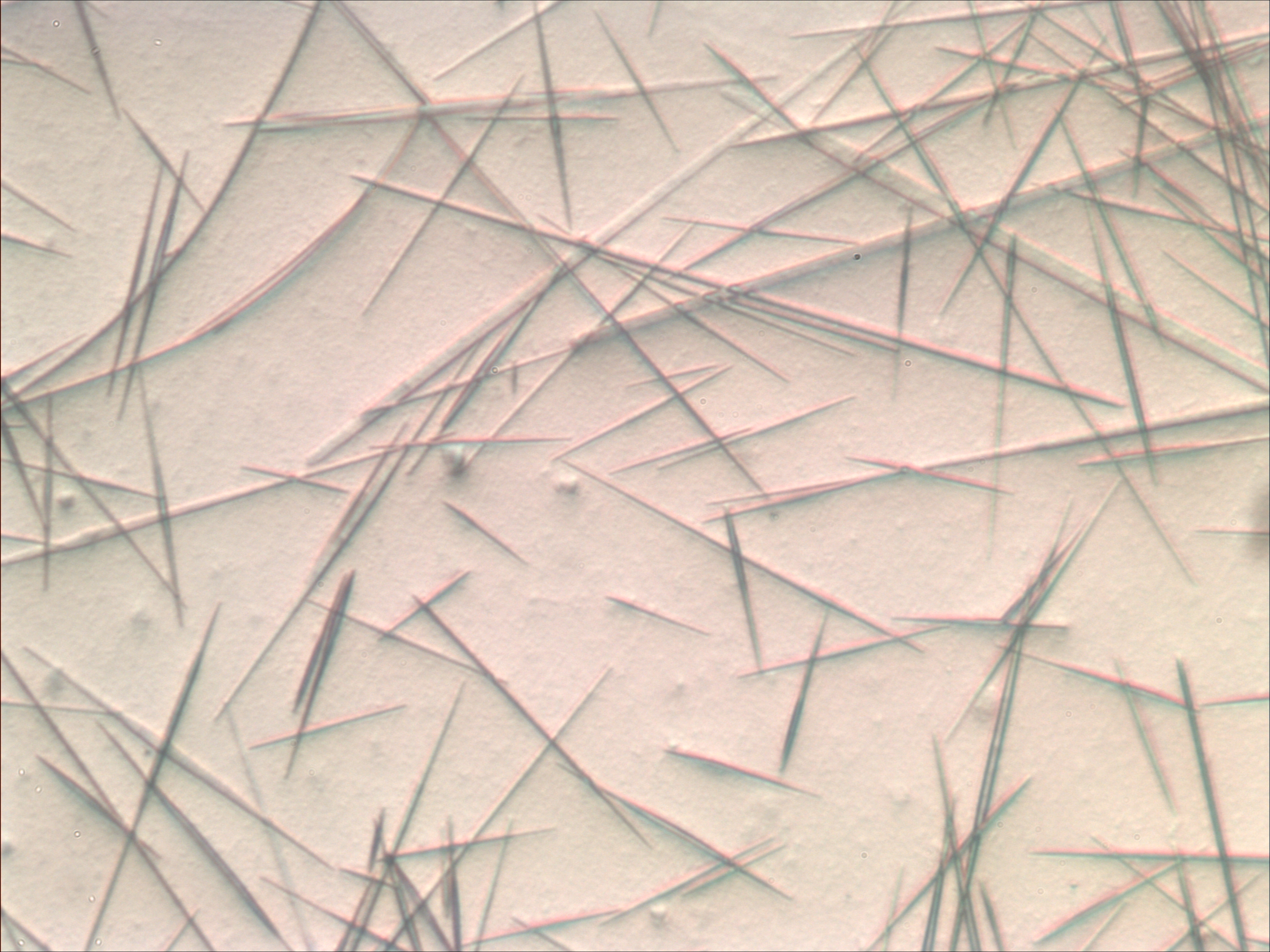
Dendrite (metal)
A dendrite in metallurgy is a characteristic tree-like structure of crystals growing as molten metal solidifies, the shape produced by faster growth along energetically favourable crystallographic directions. This dendritic growth has large consequences in regard to material properties. Formation Dendrites form in unary (one-component) systems as well as multi-component systems. The requirement is that the liquid (the molten material) be undercooled, aka supercooled, below the freezing point of the solid. Initially, a spherical solid nucleus grows in the undercooled melt. As the sphere grows, the spherical morphology becomes unstable and its shape becomes perturbed. The solid shape begins to express the preferred growth directions of the crystal. This growth direction may be due to anisotropy in the surface energy of the solid–liquid interface, or to the ease of attachment of atoms to the interface on different crystallographic planes, or both (for an example of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |