|
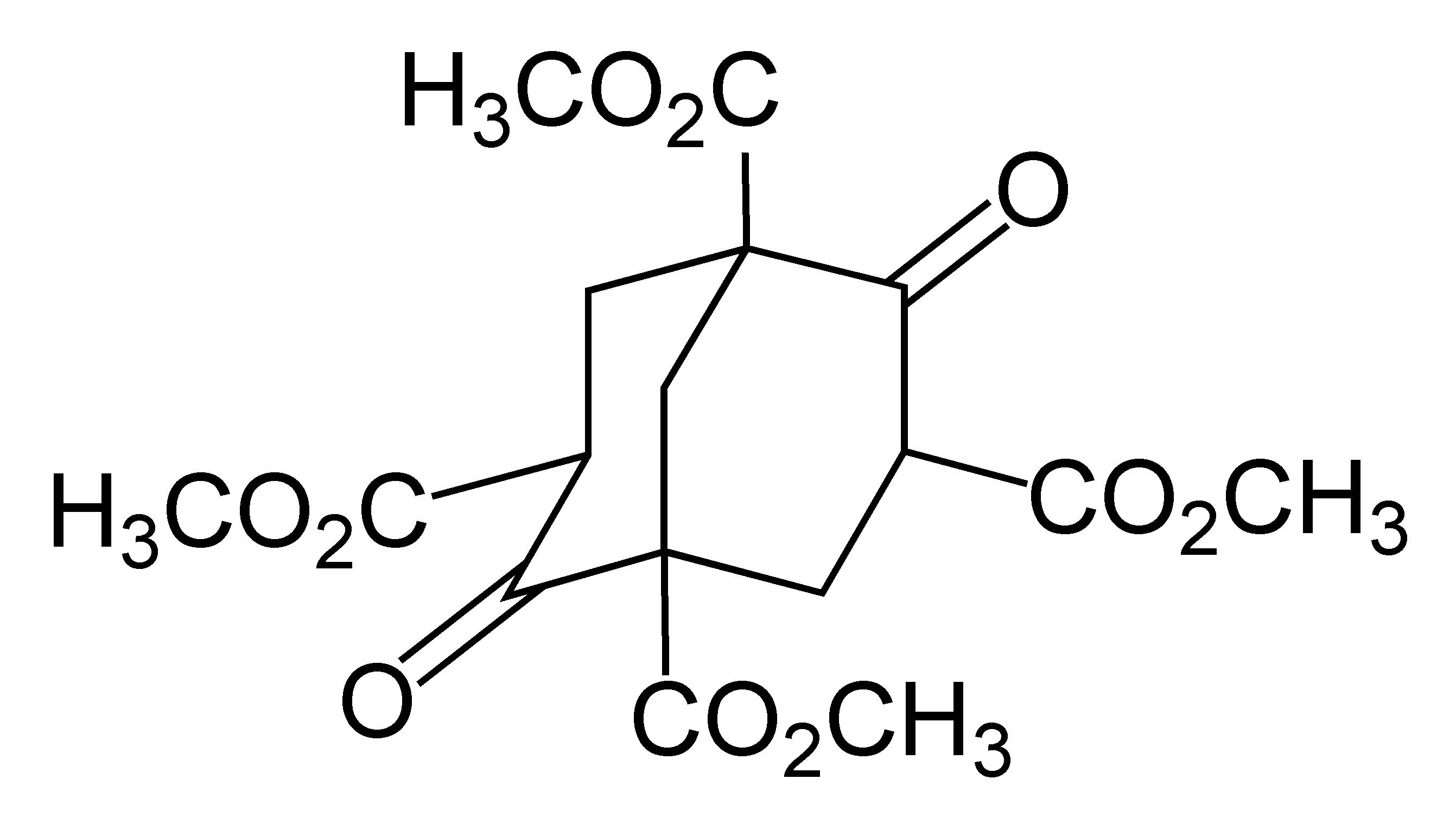
Adamantane
Adamantane is an organic compound with a formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the most stable isomer of C10H16. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal. This similarity led to the name ''adamantane'', which is derived from the Greek ''adamantinos'' (relating to steel or diamond). It is a white solid with a camphor-like odor. It is the simplest diamondoid. The discovery of adamantane in petroleum in 1933 launched a new field of chemistry dedicated to the synthesis and properties of polyhedral organic compounds. Adamantane derivatives have found practical application as drugs, polymeric materials, and thermally stable lubricants. History and synthesis In 1924, H. Decker suggested the existence of adamantane, which he called decaterpene. The first attempted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rimantadine
Rimantadine (INN, sold under the trade name 'Flumadine'') is an orally administered antiviral drug used to treat, and in rare cases prevent, influenzavirus A infection. When taken within one to two days of developing symptoms, rimantadine can shorten the duration and moderate the severity of influenza. Rimantadine can mitigate symptoms, including fever. Both rimantadine and the similar drug amantadine are derivates of adamantane. Rimantadine is found to be more effective than amantadine because when used the patient displays fewer symptoms. Rimantadine was approved by the Food and Drug Administration (FDA) in 1994. Rimantadine was approved for medical use in 1993. Seasonal H3N2 and 2009 pandemic flu samples tested have shown resistance to rimantadine, and it is no longer recommended to prescribe for treatment of the flu. Medical use Influenza A Rimantadine inhibits influenza activity by binding to amino acids in the M2 transmembrane channel and blocking proton transport ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamondoid
In chemistry, diamondoids are variants of the carbon cage molecule known as adamantane (C10H16), the smallest unit cage structure of the diamond crystal lattice. Diamondoids also known as nanodiamonds or condensed adamantanes may include one or more cages (adamantane, diamantane, triamantane, and higher polymantanes) as well as numerous isomeric and structural variants of adamantanes and polymantanes. These diamondoids occur naturally in petroleum deposits and have been extracted and purified into large pure crystals of polymantane molecules having more than a dozen adamantane cages per molecule. These species are of interest as molecular approximations of the diamond cubic framework, terminated with C−H bonds. Cyclohexamantane may be thought of as a nanometer-sized diamond of approximately . Examples Examples include: * Adamantane (C10H16) * Iceane (C12H18) * BC-8 (C14H20) * Diamantane (C14H20) also ''diadamantane'', two face-fused cages * Triamantane (C18H24), also ''tr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicyclopentadiene
Dicyclopentadiene, abbreviated DCPD, is a chemical compound with formula C10H12. At room temperature, it is a white brittle wax, although lower purity samples can be straw coloured liquids. The pure material smells somewhat of soy wax or camphor, with less pure samples possessing a stronger acrid odor. Its energy density is 10,975 Wh/l. Dicyclopentadiene is a co-produced in large quantities in the steam cracking of naphtha and gas oils to ethylene. The major use is in resins, particularly, unsaturated polyester resins. It is also used in inks, adhesives, and paints. The top seven suppliers worldwide together had an annual capacity in 2001 of 179 kilotonnes (395 million pounds). Synthesis and structure The spontaneous dimerization of cyclopentadiene at room temperature to form dicyclopentadiene proceeds to around 50% conversion over 24 hours and yields the ''endo'' isomer in better than 99:1 ratio as the kinetically favored product (about 150:1 ''endo'':''exo'' at 80 °C). H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amantadine
Amantadine, sold under the brand name Gocovri among others, is a medication used to treat dyskinesia associated with parkinsonism and influenza caused by type A influenzavirus, though its use for the latter is no longer recommended due to widespread drug resistance. It acts as a nicotinic antagonist, dopamine agonist, and noncompetitive NMDA antagonist. The antiviral mechanism of action is antagonism of the influenzavirus A M2 proton channel, which prevents endosomal escape (i.e. the release of viral genetic material into the host cytoplasm). Amantadine was first used for the treatment of influenza A. After antiviral properties were initially reported in 1963, amantadine received approval for prophylaxis against the influenza virus A in 1976. However, amantadine-resistant influenza viruses were first reported during the 1980 influenza A epidemic and resistance frequency continued to rise into the early 2000s. Currently, amantadine is no longer recommended for the treatme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Memantine
Memantine is a medication used to slow the progression of moderate-to-severe Alzheimer's disease. It is taken by mouth. Common side effects include headache, constipation, sleepiness, and dizziness. Severe side effects may include blood clots, psychosis, and heart failure. It is believed to work by acting on NMDA receptors, working as pore blockers of these ion channels. Memantine was approved for medical use in the United States in 2003. It is available as a generic medication. In 2020, it was the 152nd most commonly prescribed medication in the United States, with more than 3million prescriptions. Medical use Alzheimer's disease and dementia Memantine is used to treat moderate-to-severe Alzheimer's disease, especially for people who are intolerant of or have a contraindication to AChE (acetylcholinesterase) inhibitors.NICE review of technology appraisal guidance 111 January 18, 201Alzheimer's disease - donepezil, galantamine, rivastigmine and memantine (review): fin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vladimir Prelog
Vladimir Prelog (23 July 1906 – 7 January 1998) was a Croatian-Swiss organic chemist who received the 1975 Nobel Prize in chemistry for his research into the stereochemistry of organic molecules and reactions. Prelog was born and grew up in Sarajevo.Vladimir Prelog (1975Autobiography the Nobel Committee. He lived and worked in Prague, Zagreb and Zürich during his lifetime. Early life Prelog was born in Sarajevo, Condominium of Bosnia and Herzegovina, at that time within Austria-Hungary, to Croat parents who were working there. His father, Milan, a native of Zagreb, was a history professor at a gymnasium in Sarajevo and later at the University of Zagreb. As an 8-year-old boy, he stood near the place where the assassination of Franz Ferdinand occurred. Education Prelog attended elementary school in Sarajevo, but in 1915, as a child, Prelog moved to Zagreb (then part of the Austro-Hungary) with his parents. In Zagreb he graduated from elementary school. At first, he attende ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society (professional association) in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 34,000 in the UK and a further 8,000 abroad. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adams' Catalyst
Adams' catalyst, also known as platinum dioxide, is usually represented as platinum(IV) oxide Water of crystallization, hydrate, PtO2•H2O. It is a catalyst for hydrogenation and hydrogenolysis in organic synthesis. This dark brown powder is commercially available. The oxide itself is not an active catalyst, but it becomes active after exposure to hydrogen whereupon it converts to platinum black, which is responsible for reactions. Preparation Adams' catalyst is prepared from chloroplatinic acid H2PtCl6 or ammonium chloroplatinate, (NH4)2PtCl6, by fusion with sodium nitrate. The first published preparation was reported by V. Voorhees and Roger Adams. The procedure involves first preparing a platinum nitrate which is then heated to expel nitrogen oxides. :H2PtCl6 + 6 NaNO3 → Pt(NO3)4 + 6 NaCl (aq) + 2 HNO3 :Pt(NO3)4 → PtO2 + 4 NO2 + O2 The resulting brown cake is washed with water to free it from nitrates. The catalyst can either be used as is or dried and stored in a desicca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paul Von Ragué Schleyer
Paul von Ragué Schleyer (February 27, 1930 – November 21, 2014) was an American physical organic chemist whose research is cited with great frequency. A 1997 survey indicated that Dr. Schleyer was, at the time, the world's third most cited chemist, with over 1100 technical papers produced. He was Eugene Higgins Professor of Chemistry at Princeton University, Professor and co-director of the Institute for Organic Chemistry (Institut für organische Chemie) at the University of Erlangen–Nuremberg in Germany, and later Graham Perdue Professor of Chemistry at the University of Georgia in Athens, Georgia. He published twelve books in the fields of lithium chemistry, ab initio molecular orbital theory and carbonium ions. He was past president of the World Association of Theoretically Oriented Chemists, a fellow of the International Academy of Quantum Molecular Science and editor-in-chief of the ''Encyclopedia of Computational Chemistry''. Early life Born on February 27, 1930, in C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |