|
Abecma
Idecabtagene vicleucel, sold under the brand name Abecma, is a cell-based gene therapy to treat multiple myeloma. The most common side effects include cytokine release syndrome (CRS), infections, fatigue, musculoskeletal pain, and a weakened immune system (hypogammaglobulinemia). Idecabtagene vicleucel is a B-cell maturation antigen (BCMA)-directed genetically modified autologous chimeric antigen receptor (CAR) T-cell therapy. Each dose is customized using a patient's own T-cells, which are a type of white blood cell, that are collected and genetically modified to include a new gene that facilitates targeting and killing myeloma cells, and infused back into the patient. Idecabtagene vicleucel was approved for medical use in the United States in March 2021. It is the first cell-based gene therapy approved by the US Food and Drug Administration (FDA) for the treatment of multiple myeloma. It was approved for medical use in the European Union in August 2021. Medical uses I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CAR T-cell Therapy
In biology, chimeric antigen receptors (CARs)—also known as chimeric immunoreceptors, chimeric T cell receptors or artificial T cell receptors—are receptor (biochemistry), receptor proteins that have been engineered to give T cells the new ability to target a specific antigen. The receptors are Fusion protein, chimeric in that they combine both antigen-binding and T cell activating functions into a single receptor. CAR T cell therapy uses T cells engineered with CARs to treat cancer. T cells are modified to recognize cancer cells and destroy them. The standard approach is to harvest T cells from patients, genetically alter them, then infuse the resulting CAR T cells into patients to attack their tumors. CAR T cells can be derived either autologously from T cells in a patient's own blood or Allotransplantation, allogeneically from those of a donor. Once isolated, these T cells are genetically engineered to express a specific CAR, using a vector derived from an engineered lentivi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chimeric Antigen Receptor T Cell
In biology, chimeric antigen receptors (CARs)—also known as chimeric immunoreceptors, chimeric T cell receptors or artificial T cell receptors—are receptor proteins that have been engineered to give T cells the new ability to target a specific antigen. The receptors are chimeric in that they combine both antigen-binding and T cell activating functions into a single receptor. CAR T cell therapy uses T cells engineered with CARs to treat cancer. T cells are modified to recognize cancer cells and destroy them. The standard approach is to harvest T cells from patients, genetically alter them, then infuse the resulting CAR T cells into patients to attack their tumors. CAR T cells can be derived either autologously from T cells in a patient's own blood or allogeneically from those of a donor. Once isolated, these T cells are genetically engineered to express a specific CAR, using a vector derived from an engineered lentivirus such as HIV (see Lentiviral vector in gene therapy). T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
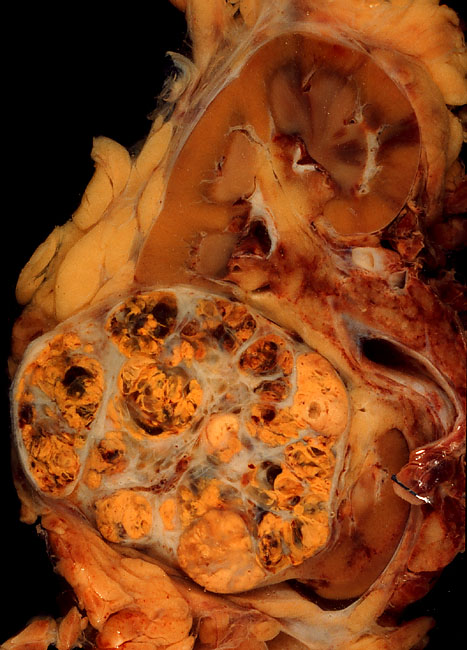
Multiple Myeloma
Multiple myeloma (MM), also known as plasma cell myeloma and simply myeloma, is a cancer of plasma cells, a type of white blood cell that normally produces antibody, antibodies. Often, no symptoms are noticed initially. As it progresses, bone pain, anemia, Kidney failure, renal insufficiency, and infections may occur. Complications may include hypercalcemia and amyloidosis. The cause of multiple myeloma is unknown. Risk factors include obesity, radiation exposure, family history, age and certain chemicals. There is an increased risk of multiple myeloma in certain occupations. This is due to the occupational exposure to aromatic hydrocarbon solvents having a role in causation of multiple myeloma. Multiple myeloma is the result of a multi-step malignant transformation, and almost universally originates from the pre-malignant stage monoclonal gammopathy of undetermined significance (MGUS). As MGUS evolves into MM, another pre-stage of the disease is reached, known as Smouldering ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bristol Myers Squibb
The Bristol-Myers Squibb Company, Trade name, doing business as Bristol Myers Squibb (BMS), is an American multinational pharmaceutical company. Headquartered in Princeton, New Jersey, BMS is one of the world's largest pharmaceutical companies and consistently ranks on the Fortune 500, ''Fortune'' 500 list of the largest U.S. corporations. For fiscal year, fiscal 2022, it had a total revenue of $46.2 billion. Bristol Myers Squibb manufactures prescription pharmaceuticals and Biopharmaceutical, biologics in several therapeutic areas, including cancer, HIV/AIDS, cardiovascular disease, diabetes, hepatitis, rheumatoid arthritis, and psychiatric disorders. BMS's primary research and development (R&D) sites are located in Lawrence Township, Mercer County, New Jersey, Lawrence, New Jersey (formerly Squibb, near Princeton), Summit, New Jersey, formerly HQ of Celgene, New Brunswick, New Jersey; Redwood City, California; and Seville in Spain, with other sites in Devens, Massachuset ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macrophage Activation Syndrome
Macrophage activation syndrome is a severe, potentially life-threatening, complication of several chronic rheumatic diseases of childhood. It occurs most commonly with systemic-onset juvenile idiopathic arthritis (SoJIA). In addition, MAS has been described in association with systemic lupus erythematosus (SLE), Kawasaki disease, and adult-onset Still's disease. It is thought to be closely related and pathophysiologically very similar to reactive (secondary) hemophagocytic lymphohistiocytosis (HLH). The incidence of MAS is unknown as there is a wide spectrum of clinical manifestations, and episodes may remain unrecognized. Signs and symptoms The hallmark clinical and laboratory features include high fever, hepatosplenomegaly, lymphadenopathy, pancytopenia, liver dysfunction, disseminated intravascular coagulation, hypofibrinogenemia, hyperferritinemia, and hypertriglyceridemia. Despite marked systemic inflammation, the erythrocyte sedimentation rate (ESR) is paradoxically d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Approved Gene Therapies
Approved may refer to: *Approved drug, a preparation that has been validated for a therapeutic use by a ruling authority of a government *''Approved'', a 2013 album by Chester Thompson Chester Thompson (born December 11, 1948) is an American drummer best known for his tenures with Frank Zappa and the Mothers of Invention, Weather Report, Santana (band), Santana, Genesis (band), Genesis and Phil Collins. Thompson has performed ... Trio * ''Approved'' (Ubiquitous Synergy Seeker album) {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drugs That Are A Gene Therapy
A drug is any chemical substance other than a nutrient or an essential dietary ingredient, which, when administered to a living organism, produces a biological effect. Consumption of drugs can be via inhalation, injection, smoking, ingestion, absorption via a patch on the skin, suppository, or dissolution under the tongue. In pharmacology, a drug is a chemical substance, typically of known structure, which, when administered to a living organism, produces a biological effect. A pharmaceutical drug, also called a medication or medicine, is a chemical substance used to treat, cure, prevent, or diagnose a disease or to promote well-being. Traditionally drugs were obtained through extraction from medicinal plants, but more recently also by organic synthesis. Pharmaceutical drugs may be used for a limited duration, or on a regular basis for chronic disorders. Classification Pharmaceutical drugs are often classified into drug classes—groups of related drugs that have simila ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cancer Treatments
Cancer treatments are a wide range of treatments available for the many different types of cancer, with each cancer type needing its own specific treatment. Treatments can include surgery, chemotherapy, radiation therapy, hormonal therapy, targeted therapy including small-molecule drugs or monoclonal antibodies, and PARP inhibitors such as olaparib. Other therapies include hyperthermia, immunotherapy, photodynamic therapy, and stem-cell therapy. Most commonly cancer treatment involves a series of separate therapies such as chemotherapy before surgery. Angiogenesis inhibitors are sometimes used to enhance the effects of immunotherapies. The choice of therapy depends upon the location and grade of the tumor and the stage of the disease, as well as the general state of the patient. Biomarker testing can help to determine the type of cancer, and indicate the best therapy. A number of experimental cancer treatments are continuously under development. In 2023 it was estimated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drugs Developed By Bristol Myers Squibb
A drug is any chemical substance other than a nutrient or an essential dietary ingredient, which, when administered to a living organism, produces a biological effect. Consumption of drugs can be via insufflation (medicine), inhalation, drug injection, injection, smoking, ingestion, absorption (skin), absorption via a dermal patch, patch on the skin, suppository, or sublingual administration, dissolution under the tongue. In pharmacology, a drug is a chemical substance, typically of known structure, which, when administered to a living organism, produces a biological effect. A pharmaceutical drug, also called a medication or medicine, is a chemical substance used to pharmacotherapy, treat, cure, preventive healthcare, prevent, or medical diagnosis, diagnose a disease or to promote well-being. Traditionally drugs were obtained through extraction from medicinal plants, but more recently also by organic synthesis. Pharmaceutical drugs may be used for a limited duration, or on a re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Celgene
Celgene Corporation, headquartered in Summit, New Jersey, was a pharmaceutical company that produced cancer and immunology drugs. Its primary products were Revlimid (lenalidomide), which is used in the treatment of multiple myeloma (63% of 2018 revenues); Pomalyst and Imnovid (Pomalidomide), also used in the treatment of multiple myeloma (13% of 2018 revenues); and Otezla (Apremilast), used in the treatment of psoriasis (11% of 2018 revenues). In 2018, 66% of the company's revenues came from the United States. In 2019, the company was acquired by Bristol Myers Squibb (BMS); as part of the acquisition, Otezla was sold to Amgen. History Celgene was originally a unit of Celanese. In 1986, Celanese completed the corporate spin-off of Celgene following the merger of Celanese with Hoechst AG, American Hoechst. In August 2000, Celgene acquired Signal Pharmaceuticals, Inc., a privately held company that developed pharmaceuticals to regulate disease-related genes. Signal Pharmaceuticals w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drug
An orphan drug is a medication, pharmaceutical agent that is developed to treat certain rare medical conditions. An orphan drug would not be profitable to produce without government assistance, due to the small population of patients affected by the conditions. The conditions that orphan drugs are used to treat are referred to as orphan diseases. The assignment of orphan status to a disease and to drugs developed to treat it is a matter of public policy that depends on the legislation (if there is any) of the country. Designation of a drug as an orphan drug has yielded medical breakthroughs that might not otherwise have been achieved, due to the economics of drug medical research, research and development. Examples of this can be that in the U.S. and the EU, it is easier to gain marketing approval for an orphan drug. There may be other financial incentives, such as an extended period of exclusivity, during which the producer has sole rights to market the drug. All are intended to en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |