|
1,1-Diphenylacetone
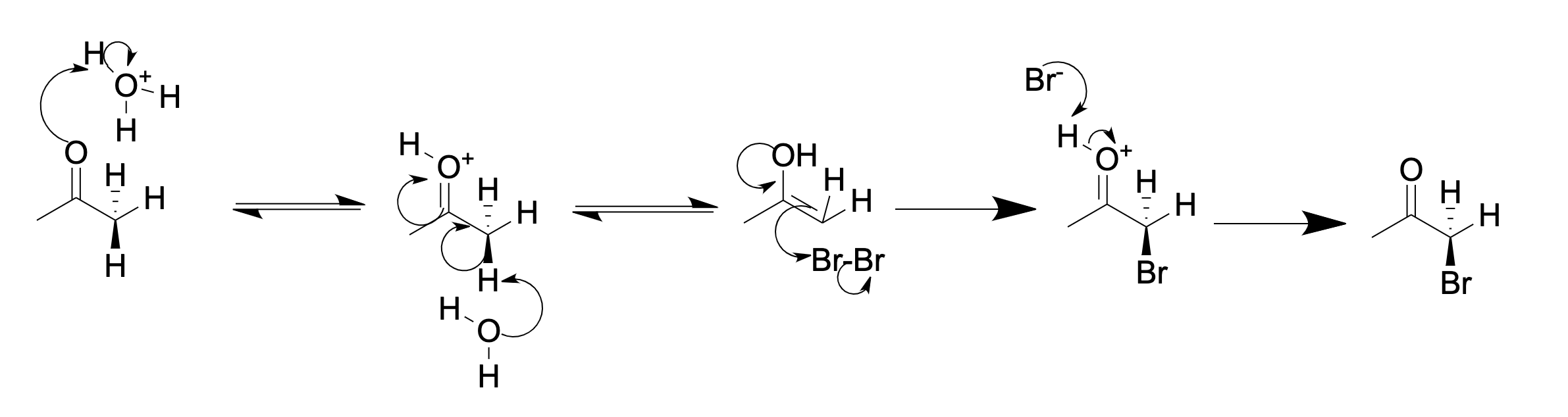
1,1-Diphenylacetone is an organic compound composed of a benzhydryl group and a methyl group attached to a central carbonyl group. Preparation One method is where phenylacetone is dissolved in benzene, reacted with bromine to effect an α-keto bromination and stirred for 3-6 hours. Then this mixture is slowly added to a solution of anhydrous aluminium chloride in benzene to catalyze a Friedel-Crafts alkylation. A lengthy workup of the reaction mixture ends in recrystallization of the product 1,1-diphenylacetone from petroleum ether. Alternative syntheses: Applications #β-Phenylmethamphetamine β-Phenylmethamphetamine (''N'',α-dimethyl-β-phenyl-phenethylamine) is a potent and long lasting stimulant drug A drug is any chemical substance other than a nutrient or an essential dietary ingredient, which, when administered to a livi ... # Diphenadione References Aromatic ketones Benzhydryl compounds {{ketone-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
β-Phenylmethamphetamine
β-Phenylmethamphetamine (''N'',α-dimethyl-β-phenyl-phenethylamine) is a potent and long lasting stimulant drug A drug is any chemical substance other than a nutrient or an essential dietary ingredient, which, when administered to a living organism, produces a biological effect. Consumption of drugs can be via insufflation (medicine), inhalation, drug i .... Synthesis 1,1-Diphenylacetone is also used in the synthesis of Diphenadione. 1,1-diphenylacetone synthesis: See also * 3-Benzhydrylmorpholine * 3,3-Diphenylcyclobutanamine * Desoxypipradrol References {{DEFAULTSORT:Phenylmethamphetamine, β- Stimulants Methamphetamines Benzhydryl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzhydryl Compounds
The benzhydryl compounds are a group of organic compounds whose parent structures include diphenylmethane (which is two benzene rings connected by a single methane), with any number of attached substituents, including bridges. This group typically excludes compounds in which either benzene is fused to another ring (bicyclic, tricyclic, polycyclic) or includes a heteroatom, or where the methane connects to three or four benzenes. The benzhydryl '' radical'' can be abbreviated or Bzh. Carboaromatic Alcohols *''Acyclic:'' pridinol *''Pyrolidino:'' diphenylprolinol *''2-Piperidine:'' pipradrol *''4-Piperidine:'' terfenadine, fexofenadine *''Benzilic ester:'' QNB, JB-336, JB-318, benactyzine Alkenes *''Tricycle:'' amitriptyline, melitracen, cyclobenzaprine, tianeptine, amineptine, clopenthixol, chlorprothixene, flupentixol, thiothixene, zuclopenthixol *''Tricyclic and piperidine:'' pimethixene, cyproheptadine *''Acyclic:'' gilutensin Alkyl(amine)s *''Acyclic:'' (3- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bonded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For exam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such as aldehydes, ketones and carboxylic acid), as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, such that carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides, chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, Hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylacetone
Phenylacetone, also known as phenyl-2-propanone, is an organic compound with the chemical formula C6H5CH2COCH3. It is a colorless oil that is soluble in organic solvents. It is a mono- substituted benzene derivative, consisting of an acetone attached to a phenyl group. As such, its systematic IUPAC name is 1-phenyl-2-propanone. This substance is used in the manufacture of methamphetamine and amphetamine, where it is commonly known as P2P. Due to illicit drug labs using phenylacetone to make amphetamines, phenylacetone was declared a schedule II controlled substance in the United States in 1980. In humans, phenylacetone occurs as a metabolite of amphetamine and methamphetamine via FMO3-mediated oxidative deamination. Synthesis There are many routes to synthesize phenylacetone. Industry uses the gas-phase ketonic decarboxylation of phenylacetic acid using acetic acid over a ceria-alumina solid acid catalyst. A related laboratory-scale reaction has been described. An alte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig (in 1825) and Antoine Jérôme Balard (in 1826), its name was derived , referring to its sharp and pungent smell. Elemental bromine is very reactive and thus does not occur as a free element in nature. Instead, it can be isolated from colourless soluble crystalline mineral halide Ionic salt, salts analogous to table salt, a property it shares with the other halogens. While it is rather rare in the Earth's crust, the high solubility of the bromide ion (Br) has caused its Bromine cycle, accumulation in the oceans. Commercially the element is easily extracted from brine evaporation ponds, mostly in the United States and Israel. The mass of bromine in the oce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone Halogenation
In organic chemistry, α-keto halogenation is a special type of halogenation. The reaction may be carried out under either acidic or basic conditions in an aqueous medium with the corresponding elemental halogen. In this way, chloride, bromide, and iodide (but notably not fluoride) functionality can be inserted selectively in the Alpha and beta carbon, alpha position of a ketone. The position alpha to the carbonyl group () in a ketone is easily halogenated. This is due to its ability to form an enolate () in basic (chemistry), basic solution, or an enol () in acidic solution. An example of alpha halogenation is the bromination, mono-bromination of acetone (), carried out under either acidic or basic conditions, to give bromoacetone: Acidic (in acetic acid): Basic (in aqueous NaOH): In acidic solution, usually only one alpha hydrogen is replaced by a halogen, as each successive halogenation is slower than the first. The halogen decreases the basicity of the carbonyl oxygen, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Chloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms a hexahydrate with the formula , containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron(III) chloride, giving them a yellow colour. The anhydrous form is commercially important. It has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium, but large amounts are also used in other areas of the chemical industry. The compound is often cited as a Lewis acid. It is an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature. Structure Anhydrous adopts three structures, depending on the temperature and the state (solid, liquid, gas). Solid has a sheet-like layered structure with cubic close-packed chloride ions. In this framework, the Al centres exhibit octahedral coordination geom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Petroleum Ether
Petroleum ether is the petroleum fraction consisting of aliphatic hydrocarbons and boiling in the range 35–60 °C, and commonly used as a laboratory solvent. Despite the name, petroleum ether is not an ether; the term is used only figuratively, signifying extreme lightness and volatility. Properties The very lightest, most volatile liquid hydrocarbon solvents that can be bought from laboratory chemical suppliers may also be offered under the name petroleum ether. Petroleum ether consists mainly of aliphatic hydrocarbons and is usually low in aromatics. It is commonly hydrodesulfurized and may be hydrogenated to reduce the amount of aromatic and other Saturated and unsaturated compounds, unsaturated hydrocarbons. Petroleum ether bears normally a descriptive suffix giving the boiling range. Thus, from the leading international laboratory chemical suppliers it is possible to buy various petroleum ethers with boiling ranges such as 30–50 °C, 40–60 °C, 50–70& ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |