|
β-Isophorone
β-Isophorone is an organic compound with the formula (CH3)3C6H7O. Classified as a β,γ-unsaturated ketone, it is an isomer of and common impurity in the major industrial intermediate α-isophorone, which is produced from acetone. Like the alpha isomer, beta-isophorone is a colorless liquid. See also *Phorone Phorone, or diisopropylidene acetone, is a yellow crystalline substance with a geranium odor, with formula or . Preparation It was first obtained in 1837 in impure form by the French chemist Auguste Laurent, who called it "camphoryle". In 1849, ... References {{DEFAULTSORT:Isophorone, β- Ketones Ketone solvents Cyclohexenes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isophorone
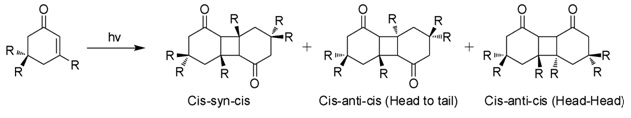
Isophorone is an Alpha-beta Unsaturated carbonyl compounds, α,β-unsaturated cyclic ketone. It is a colorless liquid with a characteristic peppermint-like odor, although commercial samples can appear yellowish. Used as a solvent and as a precursor to polymers, it is produced on a large scale industrially. Structure and reactivity Isophorone undergoes reactions characteristic of an α,β-unsaturated ketone. Hydrogenation gives the cyclohexanone derivative. Epoxidation with basic hydrogen peroxide affords the oxide. Isophorone is degraded by attack of hydroxyl radicals. Photodimerization When exposed to sunlight in aqueous solutions, isophorone undergoes 2+2 photocycloaddition to give three isomeric photodimers (Figure). These "diketomers" are cis-syn-cis, head to tail (HT), cys-anti-cys (HT), and head-head (HH). The formation of HH photodimers is favored over HT photodimers with increasing polarity of the medium. Natural occurrence Isophorone occurs naturally in cran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' are methyl), with the formula . Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, ''e.g.'', testosterone, and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered retained IUPAC names, although some introdu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the existence or possibility of isomers. Isomers do not necessarily share similar chemical property, chemical or physical property, physical properties. Two main forms of isomerism are structural isomerism, structural (or constitutional) isomerism, in which ''chemical bond, bonds'' between the atoms differ; and stereoisomerism (or spatial isomerism), in which the bonds are the same but the ''relative positions'' of the atoms differ. Isomeric relationships form a hierarchy. Two chemicals might be the same constitutional isomer, but upon deeper analysis be stereoisomers of each other. Two molecules that are the same stereoisomer as each other might be in different conformational forms or be different Isotopologue, isotopologues. The depth of analy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a characteristic pungent odor. Acetone is miscibility, miscible with properties of water, water and serves as an important organic solvent in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and for production of methyl methacrylate and bisphenol A, which are precursors to widely used plastics.Acetone World Petrochemicals report, January 2010Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. It is a common building block in organic chemistry. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phorone
Phorone, or diisopropylidene acetone, is a yellow crystalline substance with a geranium odor, with formula or . Preparation It was first obtained in 1837 in impure form by the French chemist Auguste Laurent, who called it "camphoryle". In 1849, the French chemist Charles Frédéric Gerhardt and his student Jean Pierre Liès-Bodart prepared it in a pure state and named it "phorone". On both occasions it was produced by ketonization through the dry distillation of the calcium salt of camphoric acid. : It is now typically obtained by the acid-catalysed twofold aldol condensation of three molecules of acetone. Mesityl oxide is obtained as an intermediate and can be isolated. Crude phorone can be purified by repeated recrystallization from ethanol or ether, in which it is soluble. Reactions Phorone can condense with ammonia to form triacetone amine. See also *Isophorone Isophorone is an Alpha-beta Unsaturated carbonyl compounds, α,β-unsaturated cyclic ketone. It is a c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketones
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' are methyl), with the formula . Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, ''e.g.'', testosterone, and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered retained IUPAC names, although some introd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |