|
(1,1'-Bis(diphenylphosphino)ferrocene)palladium(II) Dichloride
[1,1'‑Bis(diphenylphosphino)ferrocene]palladium(II) dichloride is a palladium complex containing the bidentate ligand 1,1'-bis(diphenylphosphino)ferrocene (dppf), abbreviated as [(dppf)PdCl]. This commercially available material can be prepared by reacting dppf with a suitable nitrile complex of palladium dichloride: :dppf + PdCl(RCN) → (dppf)PdCl + 2 RCN (RCN = acetonitrile, CHCN or benzonitrile, CHCN) The compound is popularly used for palladium-catalyzed coupling reactions, such as the Buchwald–Hartwig amination and the reductive homocoupling of aryl halides. References {{DEFAULTSORT:Bis(diphenylphosphino)ferrocene)palladium(II) dichloride, (1,1'- Palladium compounds Phosphine complexes Catalysts Ferrocenes Chloro complexes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichloromethane
Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with water, it is slightly polar, and miscible with many organic solvents.Rossberg, M. ''et al.'' (2006) "Chlorinated Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. . Occurrence Natural sources of dichloromethane include oceanic sources, macroalgae, wetlands, and volcanoes. However, the majority of dichloromethane in the environment is the result of industrial emissions. Production DCM is produced by treating either chloromethane or methane with chlorine gas at 400–500 °C. At these temperatures, both methane and chloromethane undergo a series of reactions producing progressively more chlorinated products. In this way, an estimated 400,000 tons were produced in the US, Europe, and Japan in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
John P
John is a common English name and surname: * John (given name) * John (surname) John may also refer to: New Testament Works * Gospel of John, a title often shortened to John * First Epistle of John, often shortened to 1 John * Second Epistle of John, often shortened to 2 John * Third Epistle of John, often shortened to 3 John People * John the Baptist (died c. AD 30), regarded as a prophet and the forerunner of Jesus Christ * John the Apostle (lived c. AD 30), one of the twelve apostles of Jesus * John the Evangelist, assigned author of the Fourth Gospel, once identified with the Apostle * John of Patmos, also known as John the Divine or John the Revelator, the author of the Book of Revelation, once identified with the Apostle * John the Presbyter, a figure either identified with or distinguished from the Apostle, the Evangelist and John of Patmos Other people with the given name Religious figures * John, father of Andrew the Apostle and Saint Peter * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysts
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some stage ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphine Complexes
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting fish, due to the presence of substituted phosphine and diphosphane (). With traces of present, is spontaneously flammable in air (pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a trigonal pyramidal structure. Phosphines are compounds that include and the organophosphines, which are derived from by substituting one or more hydrogen atoms with organic groups. They have the general formula . Phosphanes are saturated phosphorus hydrides of the form , such as triphosphane. Phosphine, PH3, is the smallest of the phosphines and the smallest of the phosphanes. History Philippe Gengembre (1764–1838), a student of Lavoisier, fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Palladium Compounds
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas. Palladium, platinum, rhodium, ruthenium, iridium and osmium form a group of elements referred to as the platinum group metals (PGMs). They have similar chemical properties, but palladium has the lowest melting point and is the least dense of them. More than half the supply of palladium and its congener platinum is used in catalytic converters, which convert as much as 90% of the harmful gases in automobile exhaust (hydrocarbons, carbon monoxide, and nitrogen dioxide) into nontoxic substances (nitrogen, carbon dioxide and water vapor). Palladium is also used in electronics, dentistry, medicine, hydrogen purification, chemical applications, groundwater ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aryl Halide
In organic chemistry, an aryl halide (also known as haloarene) is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications. Preparation The two main preparatory routes to aryl halides are direct halogenation and via diazonium salts. Direct halogenation In the Friedel-Crafts halogenation, Lewis acids serve as catalysts. Many metal chlorides are used, examples include iron(III) chloride or aluminium chloride. The most important aryl halide, chlorobenzene is produced by this route. Monochlorination of benzene is always accompanied by formation of the dichlorobenzene derivatives. Arenes with electron donating groups react with halogens even in the absence of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homocoupling
A coupling reaction in organic chemistry is a general term for a variety of reactions where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = main group center) reacts with an organic halide of the type R'-X with formation of a new carbon-carbon bond in the product R-R'. The most common type of coupling reaction is the cross coupling reaction. Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki were awarded the 2010 Nobel Prize in Chemistry for developing palladium-catalyzed cross coupling reactions. Broadly speaking, two types of coupling reactions are recognized: *Heterocouplings combine two different partners, such as in the Heck reaction of an alkene (RC=CH) and an alkyl halide (R'-X) to give a substituted alkene, or the Corey–House synthesis of an alkane by the reaction of a lithium diorganylcuprate (R2CuLi) with an organyl (pseudo)halide (R ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stephen L
Stephen or Steven is a common English first name. It is particularly significant to Christians, as it belonged to Saint Stephen ( grc-gre, Στέφανος ), an early disciple and deacon who, according to the Book of Acts, was stoned to death; he is widely regarded as the first martyr (or " protomartyr") of the Christian Church. In English, Stephen is most commonly pronounced as ' (). The name, in both the forms Stephen and Steven, is often shortened to Steve or Stevie. The spelling as Stephen can also be pronounced which is from the Greek original version, Stephanos. In English, the female version of the name is Stephanie. Many surnames are derived from the first name, including Stephens, Stevens, Stephenson, and Stevenson, all of which mean "Stephen's (son)". In modern times the name has sometimes been given with intentionally non-standard spelling, such as Stevan or Stevon. A common variant of the name used in English is Stephan ; related names that have found some cur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Seble Wagaw
Seble-Hiwot Wagaw is an American organic chemist who is a senior leader at AbbVie pharmaceuticals outside Chicago, IL. Background and Education Wagaw was born in Addis Ababa, Ethiopia and emigrated to the United States in 1974. Her father, Teshome Gebremichael Wagaw, was a faculty member at the University of Michigan for 28 years, and her mother is Tsehai Wolde-Tsadik. She is one of three children, with an older brother and sister. She received her Bachelor's and MS degrees in Chemistry from the University of Michigan in 1994, and a Ph.D. in organic chemistry with Stephen L. Buchwald at the Massachusetts Institute of Technology in 1999. Her research in the Buchwald lab utilized chiral complexes of Palladium to forge new carbon-nitrogen bonds on Aryl rings. Career As a Senior Director for process research and R&D at Abbott Laboratories (later AbbVie) for her entire career, Wagaw has published research on enantiomerically enriched lead molecules using Pybox ligands. She has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
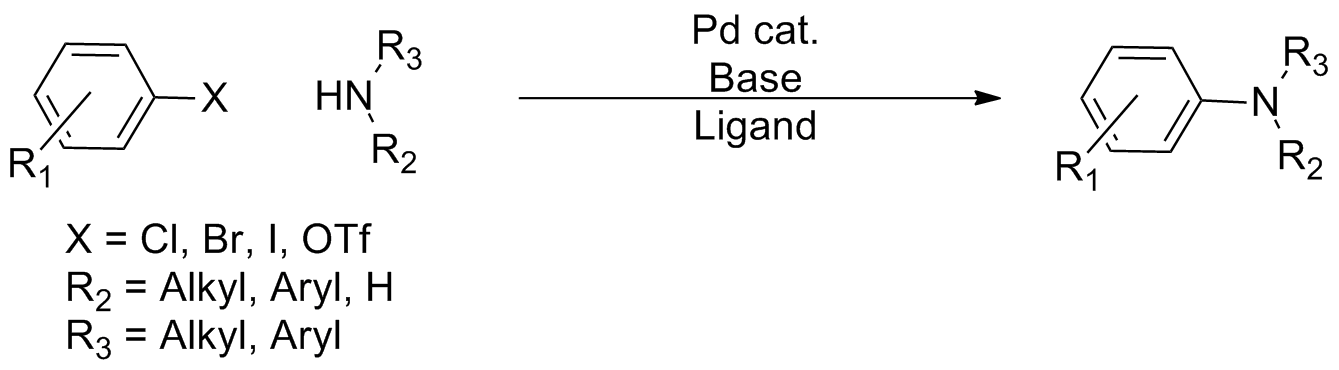
Buchwald–Hartwig Amination
In organic chemistry, the Buchwald–Hartwig amination is a chemical reaction for the synthesis of carbon–nitrogen bonds via the palladium-catalyzed coupling reactions of amines with aryl halides. Although Pd-catalyzed C-N couplings were reported as early as 1983, Stephen L. Buchwald and John F. Hartwig have been credited, whose publications between 1994 and the late 2000s established the scope of the transformation. The reaction's synthetic utility stems primarily from the shortcomings of typical methods ( nucleophilic substitution, reductive amination, etc.) for the synthesis of aromatic bonds, with most methods suffering from limited substrate scope and functional group tolerance. The development of the Buchwald–Hartwig reaction allowed for the facile synthesis of aryl amines, replacing to an extent harsher methods (the Goldberg reaction, nucleophilic aromatic substitution, etc.) while significantly expanding the repertoire of possible bond formation. : Over the co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas. Palladium, platinum, rhodium, ruthenium, iridium and osmium form a group of elements referred to as the platinum group metals (PGMs). They have similar chemical properties, but palladium has the lowest melting point and is the least dense of them. More than half the supply of palladium and its congener platinum is used in catalytic converters, which convert as much as 90% of the harmful gases in automobile exhaust (hydrocarbons, carbon monoxide, and nitrogen dioxide) into nontoxic substances (nitrogen, carbon dioxide and water vapor). Palladium is also used in electronics, dentistry, medicine, hydrogen purification, chemical applications, groundwat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


2.jpg)