nuclear transmutation on:
[Wikipedia]
[Google]
[Amazon]
 Nuclear transmutation is the conversion of one
Nuclear transmutation is the conversion of one
 Rutherford and Soddy were observing natural transmutation as a part of
Rutherford and Soddy were observing natural transmutation as a part of
The Nuclear Alchemy Gamble – Institute for Energy and Environmental Research
/ref> Technetium-99 is also produced as a waste product in
Bibnum
' lick 'à télécharger' for English version/small>. {{DEFAULTSORT:Nuclear Transmutation Nuclear physics Nuclear chemistry Radioactivity
chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
or an isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
into another chemical element. Nuclear transmutation occurs in any process where the number of protons
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' ( elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an electron (the pro ...
or neutrons in the nucleus of an atom is changed.
A transmutation can be achieved either by nuclear reaction
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two atomic nucleus, nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a t ...
s (in which an outside particle reacts with a nucleus) or by radioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
, where no outside cause is needed.
Natural transmutation by stellar nucleosynthesis
In astrophysics, stellar nucleosynthesis is the creation of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. As a ...
in the past created most of the heavier chemical elements in the known existing universe, and continues to take place to this day, creating the vast majority of the most common elements in the universe, including helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
, oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
and carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
. Most stars carry out transmutation through fusion reactions involving hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
and helium, while much larger stars are also capable of fusing heavier elements up to iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
late in their evolution.
Elements heavier than iron, such as gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
or lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
, are created through elemental transmutations that can naturally occur in supernova
A supernova (: supernovae or supernovas) is a powerful and luminous explosion of a star. A supernova occurs during the last stellar evolution, evolutionary stages of a massive star, or when a white dwarf is triggered into runaway nuclear fusion ...
e. One goal of alchemy, the transmutation of base substances into gold, is now known to be impossible by chemical means but possible by physical means. As stars begin to fuse heavier elements, substantially less energy is released from each fusion reaction. This continues until it reaches iron which is produced by an endothermic
An endothermic process is a chemical or physical process that absorbs heat from its surroundings. In terms of thermodynamics, it is a thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, ...
reaction consuming energy. No heavier element can be produced in such conditions.
One type of natural transmutation observable in the present occurs when certain radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
elements present in nature spontaneously decay by a process that causes transmutation, such as alpha
Alpha (uppercase , lowercase ) is the first letter of the Greek alphabet. In the system of Greek numerals, it has a value of one. Alpha is derived from the Phoenician letter ''aleph'' , whose name comes from the West Semitic word for ' ...
or beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
. An example is the natural decay of potassium-40
Potassium-40 (K) is a long lived and the main naturally occurring radioactive isotope of potassium. Its half-life is 1.25 billion years. It makes up about 0.012% (120 parts-per notation, ppm) of natural potassium.
Potassium-40 undergoes four dif ...
to argon-40, which forms most of the argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
in the air. Also on Earth, natural transmutations from the different mechanisms of ''natural nuclear reaction
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two atomic nucleus, nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a t ...
s'' occur, due to cosmic ray
Cosmic rays or astroparticles are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the ...
bombardment of elements (for example, to form carbon-14
Carbon-14, C-14, C or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic matter is the basis of the radiocarbon dating method pioneered by Willard Libby and coll ...
), and also occasionally from natural neutron bombardment (for example, see natural nuclear fission reactor).
Artificial transmutation may occur in machinery that has enough energy to cause changes in the nuclear structure of the elements. Such machines include particle accelerator
A particle accelerator is a machine that uses electromagnetic fields to propel electric charge, charged particles to very high speeds and energies to contain them in well-defined particle beam, beams. Small accelerators are used for fundamental ...
s and tokamak
A tokamak (; ) is a device which uses a powerful magnetic field generated by external magnets to confine plasma (physics), plasma in the shape of an axially symmetrical torus. The tokamak is one of several types of magnetic confinement fusi ...
reactors. Conventional fission power reactors also cause artificial transmutation, not from the power of the machine, but by exposing elements to neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s produced by fission from an artificially produced nuclear chain reaction. For instance, when a uranium atom is bombarded with slow neutrons, fission takes place. This releases, on average, three neutrons and a large amount of energy. The released neutrons then cause fission of other uranium atoms, until all of the available uranium is exhausted. This is called a chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.
Chain reactions are one way that sys ...
.
Artificial nuclear transmutation has been considered as a possible mechanism for reducing the volume and hazard of radioactive waste
Radioactive waste is a type of hazardous waste that contains radioactive material. It is a result of many activities, including nuclear medicine, nuclear research, nuclear power generation, nuclear decommissioning, rare-earth mining, and nuclear ...
.
History
Alchemy
The term ''transmutation'' dates back toalchemy
Alchemy (from the Arabic word , ) is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practised in China, India, the Muslim world, and Europe. In its Western form, alchemy is first ...
. Alchemists pursued the philosopher's stone, capable of chrysopoeia – the transformation of base metals into gold. While alchemists often understood chrysopoeia as a metaphor for a mystical or religious process, some practitioners adopted a literal interpretation and tried to make gold through physical experimentation. The impossibility of the metallic transmutation had been debated amongst alchemists, philosophers and scientists since the Middle Ages. Pseudo-alchemical transmutation was outlawed and publicly mocked beginning in the fourteenth century. Alchemists like Michael Maier and Heinrich Khunrath wrote tracts exposing fraudulent claims of gold making. By the 1720s, there were no longer any respectable figures pursuing the physical transmutation of substances into gold. Antoine Lavoisier
Antoine-Laurent de Lavoisier ( ; ; 26 August 17438 May 1794), When reduced without charcoal, it gave off an air which supported respiration and combustion in an enhanced way. He concluded that this was just a pure form of common air and that i ...
, in the 18th century, replaced the alchemical theory of elements with the modern theory of chemical elements, and John Dalton
John Dalton (; 5 or 6 September 1766 – 27 July 1844) was an English chemist, physicist and meteorologist. He introduced the atomic theory into chemistry. He also researched Color blindness, colour blindness; as a result, the umbrella term ...
further developed the notion of atoms (from the alchemical theory of corpuscles) to explain various chemical processes. The disintegration of atoms is a distinct process involving much greater energies than could be achieved by alchemists.
Modern physics
It was first consciously applied to modern physics by Frederick Soddy when he, along withErnest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson (30 August 1871 – 19 October 1937) was a New Zealand physicist who was a pioneering researcher in both Atomic physics, atomic and nuclear physics. He has been described as "the father of nu ...
in 1901, discovered that radioactive thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
was converting itself into radium
Radium is a chemical element; it has chemical symbol, symbol Ra and atomic number 88. It is the sixth element in alkaline earth metal, group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, ...
. At the moment of realization, Soddy later recalled, he shouted out: "Rutherford, this is transmutation!" Rutherford snapped back, "For Christ's sake, Soddy, don't call it ''transmutation''. They'll have our heads off as alchemists."
 Rutherford and Soddy were observing natural transmutation as a part of
Rutherford and Soddy were observing natural transmutation as a part of radioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
of the alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus). The parent nucleus transforms or "decays" into a daughter product, with a mass number that is reduced by four and an a ...
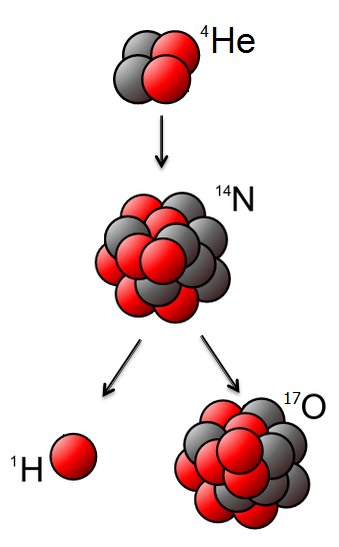
type. The first artificial transmutation was accomplished in 1925 by Patrick Blackett, a research fellow working under Rutherford, with the transmutation of nitrogen into oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, using alpha particles directed at nitrogen 14N + α → 17O + p. Rutherford had shown in 1919 that a proton (he called it a hydrogen atom) was emitted from alpha bombardment experiments but he had no information about the residual nucleus. Blackett's 1921–1924 experiments provided the first experimental evidence of an artificial nuclear transmutation reaction. Blackett correctly identified the underlying integration process and the identity of the residual nucleus. In 1932, a fully artificial nuclear reaction and nuclear transmutation was achieved by Rutherford's colleagues John Cockcroft and Ernest Walton, who used artificially accelerated protons against lithium-7 to split the nucleus into two alpha particles. The feat was popularly known as "splitting the atom", although it was not the modern nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactiv ...
reaction discovered in 1938 by Otto Hahn
Otto Hahn (; 8 March 1879 – 28 July 1968) was a German chemist who was a pioneer in the field of radiochemistry. He is referred to as the father of nuclear chemistry and discoverer of nuclear fission, the science behind nuclear reactors and ...
, Lise Meitner
Elise Lise Meitner ( ; ; 7 November 1878 – 27 October 1968) was an Austrian-Swedish nuclear physicist who was instrumental in the discovery of nuclear fission.
After completing her doctoral research in 1906, Meitner became the second woman ...
and their assistant Fritz Strassmann in heavy elements. In 1941, Rubby Sherr, Kenneth Bainbridge
Kenneth Tompkins Bainbridge (July 27, 1904 – July 14, 1996) was an American physicist at Harvard University who worked on cyclotron research. His accurate measurements of mass differences between nuclear isotopes allowed him to confirm Albert ...
and Herbert Lawrence Anderson reported the nuclear transmutation of mercury into gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
.
Later in the twentieth century the transmutation of elements within stars was elaborated, accounting for the relative abundance of heavier elements in the universe. Save for the first five elements, which were produced in the Big Bang and other cosmic ray
Cosmic rays or astroparticles are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the ...
processes, stellar nucleosynthesis accounted for the abundance of all elements heavier than boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
. In their 1957 paper '' Synthesis of the Elements in Stars'', William Alfred Fowler
William Alfred Fowler (August 9, 1911 March 14, 1995) was an American nuclear physicist, later astrophysicist, who, with Subrahmanyan Chandrasekhar, was awarded the 1983 Nobel Prize in Physics. He is known for his theoretical and experimental r ...
, Margaret Burbidge, Geoffrey Burbidge, and Fred Hoyle
Sir Fred Hoyle (24 June 1915 – 20 August 2001) was an English astronomer who formulated the theory of stellar nucleosynthesis and was one of the authors of the influential B2FH paper, B2FH paper. He also held controversial stances on oth ...
explained how the abundances of essentially all but the lightest chemical elements could be explained by the process of nucleosynthesis
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons (protons and neutrons) and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in ...
in stars.
Transmutation of other elements into gold
The alchemical tradition sought to turn the "base metal", lead, into gold. As a nuclear transmutation, it requires far less energy to turn gold into lead; for example, this would occur vianeutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, wh ...
and beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
if gold were left in a nuclear reactor for a sufficiently long period of time. In 1980, Glenn Seaborg, K. Aleklett, and a team at Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory (LBNL, Berkeley Lab) is a Federally funded research and development centers, federally funded research and development center in the Berkeley Hills, hills of Berkeley, California, United States. Established i ...
's Bevatron
The Bevatron was a particle accelerator — specifically, a Weak focusing, weak-focusing proton synchrotron — located at Lawrence Berkeley National Laboratory, U.S., which began operations in 1954. The antiproton was discovered there in ...
succeeded in producing a minuscule amount of gold from bismuth, at a net energy loss.
In 2002 and 2004, CERN
The European Organization for Nuclear Research, known as CERN (; ; ), is an intergovernmental organization that operates the largest particle physics laboratory in the world. Established in 1954, it is based in Meyrin, western suburb of Gene ...
scientists at the Super Proton Synchrotron
The Super Proton Synchrotron (SPS) is a particle accelerator of the synchrotron type at CERN. It is housed in a circular tunnel, in circumference, straddling the border of France and Switzerland near Geneva, Switzerland.
History
The SPS was d ...
reported producing a minuscule amount of gold nuclei from induced photon emissions within deliberate near-miss collisions of lead nuclei. In 2022, CERN's ISOLDE team reported producing 18 gold nuclei from proton bombardment of a uranium target. In 2025, CERN's ALICE experiment team announced that over the previous decade, they had used the Large Hadron Collider
The Large Hadron Collider (LHC) is the world's largest and highest-energy particle accelerator. It was built by the CERN, European Organization for Nuclear Research (CERN) between 1998 and 2008, in collaboration with over 10,000 scientists, ...
to replicate the 2002 SPS mechanisms at higher energies. A total of roughly 260 billion gold nuclei were created over three experiment runs, a miniscule amount massing about 90 picograms.
Transmutation in the universe
TheBig Bang
The Big Bang is a physical theory that describes how the universe expanded from an initial state of high density and temperature. Various cosmological models based on the Big Bang concept explain a broad range of phenomena, including th ...
is thought to be the origin of the hydrogen (including all deuterium
Deuterium (hydrogen-2, symbol H or D, also known as heavy hydrogen) is one of two stable isotopes of hydrogen; the other is protium, or hydrogen-1, H. The deuterium nucleus (deuteron) contains one proton and one neutron, whereas the far more c ...
) and helium in the universe. Hydrogen and helium together account for 98% of the mass of ordinary matter in the universe, while the other 2% makes up everything else. The Big Bang also produced small amounts of lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the ...
, beryllium
Beryllium is a chemical element; it has Symbol (chemistry), symbol Be and atomic number 4. It is a steel-gray, hard, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with ...
and perhaps boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
. More lithium, beryllium and boron were produced later, in a natural nuclear reaction, cosmic ray spallation.
Stellar nucleosynthesis
In astrophysics, stellar nucleosynthesis is the creation of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. As a ...
is responsible for all of the other elements occurring naturally in the universe as stable isotopes and primordial nuclide, from carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
to uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
. These occurred after the Big Bang, during star formation. Some lighter elements from carbon to iron were formed in stars and released into space by asymptotic giant branch
The asymptotic giant branch (AGB) is a region of the Hertzsprung–Russell diagram populated by evolved cool luminous stars. This is a period of stellar evolution undertaken by all low- to intermediate-mass stars (about 0.5 to 8 solar masses) lat ...
(AGB) stars. These are a type of red giant that "puffs" off its outer atmosphere, containing some elements from carbon to nickel and iron. Nuclides with mass number
The mass number (symbol ''A'', from the German word: ''Atomgewicht'', "atomic weight"), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is appro ...
greater than 64 are predominantly produced by neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, wh ...
processes—the ''s''-process and ''r''-process–in supernova
A supernova (: supernovae or supernovas) is a powerful and luminous explosion of a star. A supernova occurs during the last stellar evolution, evolutionary stages of a massive star, or when a white dwarf is triggered into runaway nuclear fusion ...
explosions and neutron star merger
A neutron star merger is the stellar collision of neutron stars. When two neutron stars fall into mutual orbit, they gradually inspiral, spiral inward due to the loss of energy emitted as gravitational radiation. When they finally meet, their me ...
s.
The Solar System
The Solar SystemCapitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Sola ...
is thought to have condensed approximately 4.6 billion years before the present, from a cloud of hydrogen and helium containing heavier elements in dust grains formed previously by a large number of such stars. These grains contained the heavier elements formed by transmutation earlier in the history of the universe.
All of these natural processes of transmutation in stars are continuing today, in our own galaxy and in others. Stars fuse hydrogen and helium into heavier and heavier elements (up to iron), producing energy. For example, the observed light curves of supernova stars such as SN 1987A show them blasting large amounts (comparable to the mass of Earth) of radioactive nickel and cobalt into space. However, little of this material reaches Earth. Most natural transmutation on the Earth today is mediated by cosmic rays
Cosmic rays or astroparticles are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the Solar ...
(such as production of carbon-14
Carbon-14, C-14, C or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic matter is the basis of the radiocarbon dating method pioneered by Willard Libby and coll ...
) and by the radioactive decay of radioactive primordial nuclides left over from the initial formation of the Solar System (such as potassium-40
Potassium-40 (K) is a long lived and the main naturally occurring radioactive isotope of potassium. Its half-life is 1.25 billion years. It makes up about 0.012% (120 parts-per notation, ppm) of natural potassium.
Potassium-40 undergoes four dif ...
, uranium and thorium), plus the radioactive decay of products of these nuclides (radium, radon, polonium, etc.). See decay chain
In nuclear science a decay chain refers to the predictable series of radioactive disintegrations undergone by the nuclei of certain unstable chemical elements.
Radioactive isotopes do not usually decay directly to stable isotopes, but rather ...
.
Artificial transmutation of nuclear waste
Overview
Transmutation of transuranium elements (i.e. actinides minusactinium
Actinium is a chemical element; it has chemical symbol, symbol Ac and atomic number 89. It was discovered by Friedrich Oskar Giesel in 1902, who gave it the name ''emanium''; the element got its name by being wrongly identified with a substa ...
to uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
) such as the isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
s of plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
(about 1wt% in the light water reactors' used nuclear fuel
Nuclear fuel refers to any substance, typically fissile material, which is used by nuclear power stations or other atomic nucleus, nuclear devices to generate energy.
Oxide fuel
For fission reactors, the fuel (typically based on uranium) is ...
or the minor actinides (MAs, i.e. neptunium
Neptunium is a chemical element; it has chemical symbol, symbol Np and atomic number 93. A radioactivity, radioactive actinide metal, neptunium is the first transuranic element. It is named after Neptune, the planet beyond Uranus in the Solar Syste ...
, americium
Americium is a synthetic element, synthetic chemical element; it has Chemical symbol, symbol Am and atomic number 95. It is radioactive and a transuranic member of the actinide series in the periodic table, located under the lanthanide element e ...
, and curium), about 0.1wt% each in light water reactors' used nuclear fuel) has the potential to help solve some problems posed by the management of radioactive waste
Radioactive waste is a type of hazardous waste that contains radioactive material. It is a result of many activities, including nuclear medicine, nuclear research, nuclear power generation, nuclear decommissioning, rare-earth mining, and nuclear ...
by reducing the proportion of long-lived isotopes it contains. (This does not rule out the need for a deep geological repository for high level radioactive waste.) When irradiated with fast neutrons in a nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
, these isotopes can undergo nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactiv ...
, destroying the original actinide
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
isotope and producing a spectrum of radioactive and nonradioactive fission products.
Ceramic targets containing actinides can be bombarded with neutrons to induce transmutation reactions to remove the most difficult long-lived species. These can consist of actinide-containing solid solutions such as , , , , or just actinide phases such as , , , mixed with some inert phases such as , , , and . The role of non-radioactive inert phases is mainly to provide stable mechanical behaviour to the target under neutron irradiation.
There are issues with this P&T (partitioning and transmutation) strategy however:
* it is limited by the costly and cumbersome need to separate long-lived fission product isotopes before they can undergo transmutation.
* some long-lived fission products, including the nuclear waste product caesium-137, are unable to capture enough neutrons for effective transmutation to occur due to their small neutron cross-section and resultingly low capture rate.
The new study led by Satoshi Chiba at Tokyo Tech (called "Method to Reduce Long-lived Fission Products by Nuclear Transmutations with Fast Spectrum Reactors") shows that effective transmutation of long-lived fission products can be achieved in fast spectrum reactors without the need for isotope separation. This can be achieved by adding a yttrium deuteride moderator.
Reactor types
For instance, plutonium can be reprocessed into mixed oxide fuels and transmuted in standard reactors. However, this is limited by the accumulation ofplutonium-240
Plutonium-240 ( or Pu-240) is an isotope of plutonium formed when plutonium-239 captures a neutron. The detection of its spontaneous fission led to its discovery in 1944 at Los Alamos and had important consequences for the Manhattan Project.
...
in spent MOX fuel, which is neither particularly fertile (transmutation to fissile plutonium-241 does occur, but at lower rates than production of more plutonium-240 from neutron capture by plutonium-239) nor fissile with thermal neutrons. Even countries like France which practice nuclear reprocessing
Nuclear reprocessing is the chemical separation of fission products and actinides from spent nuclear fuel. Originally, reprocessing was used solely to extract plutonium for producing nuclear weapons. With commercialization of nuclear power, the ...
extensively, usually do not reuse the Plutonium content of used MOX-fuel. The heavier elements could be transmuted in fast reactors, but probably more effectively in a subcritical reactor which is sometimes known as an energy amplifier and which was devised by Carlo Rubbia. Fusion neutron source
A neutron source is any device that emits neutrons, irrespective of the mechanism used to produce the neutrons. Neutron sources are used in physics, engineering, medicine, nuclear weapons, petroleum exploration, biology, chemistry, and nuclear p ...
s have also been proposed as well suited.
Fuel types
There are several fuels that can incorporate plutonium in their initial composition at their beginning of cycle and have a smaller amount of this element at the end of cycle. During the cycle, plutonium can be burnt in a power reactor, generating electricity. This process is not only interesting from a power generation standpoint, but also due to its capability of consuming the surplus weapons grade plutonium from the weapons program and plutonium resulting of reprocessing used nuclear fuel. Mixed oxide fuel is one of these. Its blend of oxides of plutonium and uranium constitutes an alternative to the low enriched uranium fuel predominantly used in light water reactors. Since uranium is present in mixed oxide, although plutonium will be burnt, second generation plutonium will be produced through the radiative capture of uranium-238 and the two subsequent beta minus decays. Fuels with plutonium andthorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
are also an option. In these, the neutrons released in the fission of plutonium are captured by thorium-232
Thorium-232 () is the main naturally occurring isotope of thorium, with a relative abundance of 99.98%. It has a half life of 14.05 billion years, which makes it the longest-lived isotope of thorium. It decays by alpha decay to radium-228; its de ...
. After this radiative capture, thorium-232 becomes thorium-233, which undergoes two beta minus decays resulting in the production of the fissile isotope uranium-233
Uranium-233 ( or U-233) is a fissile isotope of uranium that is bred from thorium-232 as part of the thorium fuel cycle. Uranium-233 was investigated for use in nuclear weapons and as a Nuclear fuel, reactor fuel. It has been used successfully ...
. The radiative capture cross section for thorium-232 is more than three times that of uranium-238, yielding a higher conversion to fissile fuel than that from uranium-238. Due to the absence of uranium in the fuel, there is no second generation plutonium produced, and the amount of plutonium burnt will be higher than in mixed oxide fuels. However, uranium-233, which is fissile, will be present in the used nuclear fuel. Weapons-grade and reactor-grade plutonium can be used in plutonium–thorium fuels, with weapons-grade plutonium being the one that shows a bigger reduction in the amount of plutonium-239.
Long-lived fission products
Some radioactive fission products can be converted into shorter-lived radioisotopes by transmutation. Transmutation of all fission products with half-life greater than one year is studied in Grenoble, with varying results.Strontium-90
Strontium-90 () is a radioactive isotope of strontium produced by nuclear fission, with a half-life of 28.79 years. It undergoes β− decay into yttrium-90, with a decay energy of 0.546 MeV. Strontium-90 has applications in medicine a ...
and caesium-137, with half-lives of about 30 years, are the largest radiation (including heat) emitters in used nuclear fuel on a scale of decades to ~305 years ( tin-121m is insignificant because of the low yield), and are not easily transmuted because they have low neutron absorption cross sections. Instead, they should simply be stored until they decay. Given that this length of storage is necessary, the fission products with shorter half-lives can also be stored until they decay.
The next longer-lived fission product is samarium-151, which has a half-life of 90 years, and is such a good neutron absorber that most of it is transmuted while the nuclear fuel is still being used; however, effectively transmuting the remaining in nuclear waste would require separation from other isotopes of samarium. Given the smaller quantities and its low-energy radioactivity, is less dangerous than and and can also be left to decay for ~970 years.
Finally, there are seven long-lived fission products. They have much longer half-lives in the range 211,000 years to 15.7 million years. Two of them, technetium-99 and iodine-129, are mobile enough in the environment to be potential dangers, are free (Technetium
Technetium is a chemical element; it has Symbol (chemistry), symbol Tc and atomic number 43. It is the lightest element whose isotopes are all radioactive. Technetium and promethium are the only radioactive elements whose neighbours in the sense ...
has no known stable isotopes) or mostly free of mixture with stable isotopes of the same element, and have neutron cross sections that are small but adequate to support transmutation.
Additionally, can substitute for uranium-238 in supplying Doppler broadening for negative feedback for reactor stability.
Most studies of proposed transmutation schemes have assumed , , and transuranium elements as the targets for transmutation, with other fission products, activation products, and possibly reprocessed uranium remaining as waste./ref> Technetium-99 is also produced as a waste product in
nuclear medicine
Nuclear medicine (nuclear radiology, nucleology), is a medical specialty involving the application of radioactivity, radioactive substances in the diagnosis and treatment of disease. Nuclear imaging is, in a sense, ''radiology done inside out'', ...
from Technetium-99m
Technetium-99m (99mTc) is a metastable nuclear isomer of technetium-99 (itself an isotope of technetium), symbolized as 99mTc, that is used in tens of millions of medical diagnostic procedures annually, making it the most commonly used Radiophar ...
, a nuclear isomer that decays to its ground state which has no further use. Due to the decay product of (the result of capturing a neutron) decaying with a relatively short half life to a stable isotope of ruthenium, a precious metal
Precious metals are rare, naturally occurring metallic chemical elements of high Value (economics), economic value. Precious metals, particularly the noble metals, are more corrosion resistant and less reactivity (chemistry), chemically reac ...
, there might also be some economic incentive to transmutation, if costs can be brought low enough.
Of the remaining five long-lived fission products, selenium-79, tin-126 and palladium-107 are produced only in small quantities (at least in today's thermal neutron, -burning light water reactors) and the last two should be relatively inert. The other two, zirconium-93 and caesium-135, are produced in larger quantities, but also not highly mobile in the environment. They are also mixed with larger quantities of other isotopes of the same element. Zirconium is used as cladding in fuel rods due to being virtually "transparent" to neutrons, but a small amount of is produced by neutron absorption from the regular zircalloy without much ill effect. Whether could be reused for new cladding material has not been subject of much study thus far.
See also
* Neutron activation *Nuclear power
Nuclear power is the use of nuclear reactions to produce electricity. Nuclear power can be obtained from nuclear fission, nuclear decay and nuclear fusion reactions. Presently, the vast majority of electricity from nuclear power is produced by ...
* List of nuclear waste treatment technologies
* Synthesis of precious metals
* Fertile material
References
External links
* "Radioactive change", Rutherford & Soddy article (1903), online and analyzed onBibnum
' lick 'à télécharger' for English version/small>. {{DEFAULTSORT:Nuclear Transmutation Nuclear physics Nuclear chemistry Radioactivity