Zygomycota on:
[Wikipedia]
[Google]
[Amazon]
Zygomycota, or zygote fungi, is a former
 The name ''Zygomycota'' refers to the zygosporangia characteristically formed by the members of this clade, in which resistant spherical
The name ''Zygomycota'' refers to the zygosporangia characteristically formed by the members of this clade, in which resistant spherical
 The term "spore" is used to describe a structure related to propagation and dispersal. Zygomycete spores can be formed through both sexual and asexual means. Before
The term "spore" is used to describe a structure related to propagation and dispersal. Zygomycete spores can be formed through both sexual and asexual means. Before

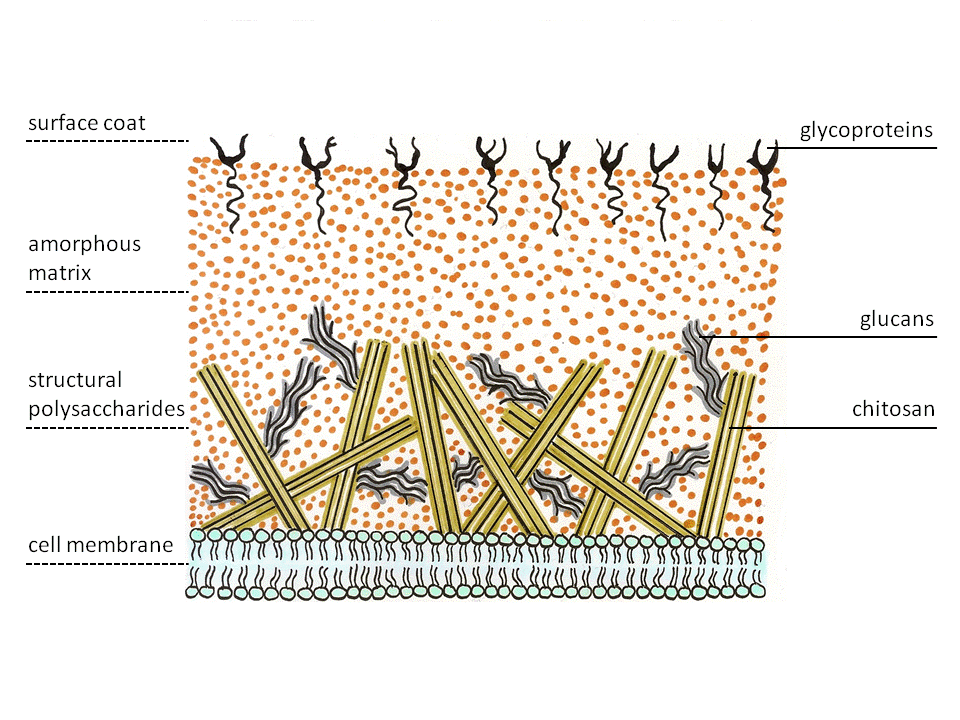 Zygomycetes exhibit a special structure of cell wall. Most fungi have
Zygomycetes exhibit a special structure of cell wall. Most fungi have


 The zygomycetes are able to grow in a wide range of environments. Most of them are mesophilic (growing at 10–40 °C with an optimum 20–35 °C), but some, like ''Mucor miehei'' or ''Mucor pusillus'', are thermophilic with a minimum growth temperature of about 20 °C and maximum extending up to 60 °C. Others like ''Mucor hiemalis'' can grow at temperatures below 0 °C.
Some species of the order Mucorales are able to grow under anaerobic conditions, while most of them require aerobic conditions. Furthermore, while the majority of the zygomycetes only grow at high water activities, some of them are able to grow in salt concentrations of at least 15%. Most species of ''Mucor'' grow rapidly on agar at room temperature filling the
The zygomycetes are able to grow in a wide range of environments. Most of them are mesophilic (growing at 10–40 °C with an optimum 20–35 °C), but some, like ''Mucor miehei'' or ''Mucor pusillus'', are thermophilic with a minimum growth temperature of about 20 °C and maximum extending up to 60 °C. Others like ''Mucor hiemalis'' can grow at temperatures below 0 °C.
Some species of the order Mucorales are able to grow under anaerobic conditions, while most of them require aerobic conditions. Furthermore, while the majority of the zygomycetes only grow at high water activities, some of them are able to grow in salt concentrations of at least 15%. Most species of ''Mucor'' grow rapidly on agar at room temperature filling the  Growth of Zygomycota in solid agar can produce low or very high fibrous colony that rapidly fills the entire Petri dish. Its color may range from pure white to shades of gray or brown. In old cultures, dark pigmented sporangia are observed. Everything depends on the species and the media used. In liquid culture, Zygomycota usually form a bland mass and do not produce spores. This is because they cannot grow aerial hyphae.
Growth of Zygomycota in solid agar can produce low or very high fibrous colony that rapidly fills the entire Petri dish. Its color may range from pure white to shades of gray or brown. In old cultures, dark pigmented sporangia are observed. Everything depends on the species and the media used. In liquid culture, Zygomycota usually form a bland mass and do not produce spores. This is because they cannot grow aerial hyphae.
Zygomycota
at the Tree of Life Web Project
Zygomycetes.org
List of all Zygomycetes species from Zygomycetes database by PM Kirk in Catalogue of Life 2008
* {{Taxonbar, from=Q215384 Fungus phyla Fungi by classification
division
Division or divider may refer to:
Mathematics
*Division (mathematics), the inverse of multiplication
*Division algorithm, a method for computing the result of mathematical division
Military
*Division (military), a formation typically consisting ...
or phylum
In biology, a phylum (; plural: phyla) is a level of classification or taxonomic rank below kingdom and above class. Traditionally, in botany the term division has been used instead of phylum, although the International Code of Nomenclature f ...
of the kingdom Fungi
A fungus ( : fungi or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and molds, as well as the more familiar mushrooms. These organisms are classified as a kingdom, separately from ...
. The members are now part of two phyla: the Mucoromycota
Mucoromycota is a division within the kingdom fungi.
They include a diverse group of various molds, including the common bread molds ''Mucor'' and ''Rhizopus''. It is a sister phylum to Dikarya. It consists of mainly mycorrhizal fungi, root end ...
and Zoopagomycota. Approximately 1060 species are known. They are mostly terrestrial in habitat, living in soil or on decaying plant or animal material. Some are parasites of plants, insects, and small animals, while others form symbiotic relationships with plants. Zygomycete hypha
A hypha (; ) is a long, branching, filamentous structure of a fungus, oomycete, or actinobacterium. In most fungi, hyphae are the main mode of vegetative growth, and are collectively called a mycelium.
Structure
A hypha consists of one or ...
e may be coenocytic
A coenocyte () is a multinucleate cell which can result from multiple nuclear divisions without their accompanying cytokinesis, in contrast to a syncytium, which results from cellular aggregation followed by dissolution of the cell membranes insid ...
, forming septa only where gamete
A gamete (; , ultimately ) is a haploid cell that fuses with another haploid cell during fertilization in organisms that reproduce sexually. Gametes are an organism's reproductive cells, also referred to as sex cells. In species that produce t ...
s are formed or to wall off dead hyphae. Zygomycota is no longer recognised as it was not believed to be truly monophyletic
In cladistics for a group of organisms, monophyly is the condition of being a clade—that is, a group of taxa composed only of a common ancestor (or more precisely an ancestral population) and all of its lineal descendants. Monophyletic gro ...
.
Etymology
 The name ''Zygomycota'' refers to the zygosporangia characteristically formed by the members of this clade, in which resistant spherical
The name ''Zygomycota'' refers to the zygosporangia characteristically formed by the members of this clade, in which resistant spherical spores
In biology, a spore is a unit of sexual or asexual reproduction that may be adapted for dispersal and for survival, often for extended periods of time, in unfavourable conditions. Spores form part of the life cycles of many plants, algae, f ...
are formed during sexual reproduction
Sexual reproduction is a type of reproduction that involves a complex life cycle in which a gamete ( haploid reproductive cells, such as a sperm or egg cell) with a single set of chromosomes combines with another gamete to produce a zygote tha ...
. ''Zygos'' is Greek
Greek may refer to:
Greece
Anything of, from, or related to Greece, a country in Southern Europe:
*Greeks, an ethnic group.
*Greek language, a branch of the Indo-European language family.
**Proto-Greek language, the assumed last common ancestor ...
for "joining" or "a yoke
A yoke is a wooden beam sometimes used between a pair of oxen or other animals to enable them to pull together on a load when working in pairs, as oxen usually do; some yokes are fitted to individual animals. There are several types of yoke, us ...
", referring to the fusion of two hyphal strands which produces these spores, and ''-mycota'' is a suffix referring to a division of fungi.
Spores
 The term "spore" is used to describe a structure related to propagation and dispersal. Zygomycete spores can be formed through both sexual and asexual means. Before
The term "spore" is used to describe a structure related to propagation and dispersal. Zygomycete spores can be formed through both sexual and asexual means. Before germination
Germination is the process by which an organism grows from a seed or spore. The term is applied to the sprouting of a seedling from a seed of an angiosperm or gymnosperm, the growth of a sporeling from a spore, such as the spores of fungi, fer ...
the spore is in a dormant state. During this period, the metabolic rate
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cell ...
is very low and it may last from a few hours to many years. There are two types of dormancy. The exogenous dormancy is controlled by environmental factors such as temperature or nutrient availability. The endogenous or constitutive dormancy depends on characteristics of the spore itself; for example, metabolic features. In this type of dormancy, germination may be prevented even if the environmental conditions favor growth.
Mitospores
In zygomycetes, mitospores ( sporangiospores) are formed asexually. They are formed in specialized structures, the mitosporangia (sporangia) that contain few to several thousand of spores, depending on the species. Mitosporangia are carried by specialized hyphae, the mitosporangiophores (sporangiophores). These specialized hyphae usually show negative gravitropism and positive phototropism allowing good spore dispersal. The sporangia wall is thin and is easily destroyed by mechanical stimuli (e.g. falling raindrops, passing animals), leading to the dispersal of the ripe mitospores. The walls of these spores containsporopollenin
270px, SEM image of pollen grains
Sporopollenin is one of the most chemically inert biological polymers. It is a major component of the tough outer (exine) walls of plant spores and pollen grains. It is chemically very stable and is usually well ...
in some species. Sporopollenin is formed out of β-carotene and is very resistant to biological and chemical degradation.
Zygomycete spores may also be classified in respect to their persistence:
Chlamydospores
Chlamydospores are asexual spores different from sporangiospores. The primary function of chlamydospores is the persistence of the mycelium and they are released when the mycelium degrades. Chlamydospores have no mechanism for dispersal. In zygomycetes the formation of chlamydospores is usually intercalar. However, it may also be terminal. In accordance with their function chlamydospores have a thick cell wall and are pigmented.
Zygophores
Zygophores are chemotropic aerial hyphae that are the sex organs of Zygomycota, except for Phycomyces in which they are not aerial but found in the substratum. They have two different mating types (+) and (-). The opposite mating types grow towards each other due to volatilepheromone
A pheromone () is a secreted or excreted chemical factor that triggers a social response in members of the same species. Pheromones are chemicals capable of acting like hormones outside the body of the secreting individual, to affect the behavio ...
s given off by the opposite strand, mainly trisporic acid
Trisporic acids (TSAs) are C-18 terpenoid compounds synthesized via β-carotene and retinol pathways in the zygomycetes. They are pheromone compound responsible for sexual differentiation in those fungal species. TSAs and related compounds make u ...
and its precursors. Once two opposite mating types have made initial contact, they give rise to a zygospore through multiple steps.
Zygospore formation is the result of a multiple step process beginning with compatible mating type zygophores growing towards each other. Once contact between the zygophores has been made, their walls adhere to each other, flatten and then the contact site is referred to as the fusion septum. The tips of the zygophore become distended and form what is called the progametangia. A septum develops by gradual inward extension until it separates the terminal gametangia from the progametangial base. At this point the zygophore is then called the suspensor. Vesicles accumulate at the fusion septum at which time it begins to dissolve. A little before the fusion septum completely dissolves, the primary outer wall begins to thicken. This can be seen as dark patches on the primary wall as the fusion septum dissolves. These dark patches on the wall will eventually develop into warty structures that make up the thickness of the zygospore wall. As the zygospore enlarges, so do the warty structures until there are contiguous around the entire cell. At this point, electron microscopy can no longer penetrate the wall. Eventually the warts push through the primary wall and darken which is likely caused by melanin
Melanin (; from el, μέλας, melas, black, dark) is a broad term for a group of natural pigments found in most organisms. Eumelanin is produced through a multistage chemical process known as melanogenesis, where the oxidation of the amino ...
.
Meiosis
Meiosis (; , since it is a reductional division) is a special type of cell division of germ cells in sexually-reproducing organisms that produces the gametes, such as sperm or egg cells. It involves two rounds of division that ultimately resu ...
usually occurs before zygospore germination and there are a few main types of distinguishable nuclear
Nuclear may refer to:
Physics
Relating to the nucleus of the atom:
* Nuclear engineering
*Nuclear physics
*Nuclear power
*Nuclear reactor
*Nuclear weapon
*Nuclear medicine
*Radiation therapy
*Nuclear warfare
Mathematics
*Nuclear space
*Nuclear ...
behavior. Type 1 is when the nuclei fuse quickly, within a few days, resulting in mature zygospore having haploid
Ploidy () is the number of complete sets of chromosomes in a cell, and hence the number of possible alleles for autosomal and pseudoautosomal genes. Sets of chromosomes refer to the number of maternal and paternal chromosome copies, respectively ...
nuclei. Type 2 is when some nuclei do not pair and degenerate instead, meiosis is delayed until germination. Type 3 is when haploid nuclei continue to divide mitotically and then some associate into groups and some do not. This results in diploid
Ploidy () is the number of complete sets of chromosomes in a cell, and hence the number of possible alleles for autosomal and pseudoautosomal genes. Sets of chromosomes refer to the number of maternal and paternal chromosome copies, respectively ...
and haploid nuclei being found in the germ sporangium
A sporangium (; from Late Latin, ) is an enclosure in which spores are formed. It can be composed of a single cell or can be multicellular. Virtually all plants, fungi, and many other lineages form sporangia at some point in their life cy ...
.
Cell wall
 Zygomycetes exhibit a special structure of cell wall. Most fungi have
Zygomycetes exhibit a special structure of cell wall. Most fungi have chitin
Chitin ( C8 H13 O5 N)n ( ) is a long-chain polymer of ''N''-acetylglucosamine, an amide derivative of glucose. Chitin is probably the second most abundant polysaccharide in nature (behind only cellulose); an estimated 1 billion tons of chit ...
as structural polysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with wa ...
, while zygomycetes synthesize chitosan
Chitosan is a linear polysaccharide composed of randomly distributed β-(1→4)-linked D-glucosamine (deacetylated unit) and ''N''-acetyl-D-glucosamine (acetylated unit). It is made by treating the chitin shells of shrimp and other crustacean ...
, the deacetylated homopolymer
A polymer (; Greek '' poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic an ...
of chitin. Chitin is built of β-1,4 bonded ''N''-acetyl glucosamine. Fungal hyphae grow at the tip. Therefore, specialized vesicles, the chitosomes, bring precursors of chitin and its synthesizing enzyme, chitin synthetase, to the outside of the membrane by exocytosis
Exocytosis () is a form of active transport and bulk transport in which a cell transports molecules (e.g., neurotransmitters and proteins) out of the cell ('' exo-'' + ''cytosis''). As an active transport mechanism, exocytosis requires the use o ...
. The enzyme on the membrane catalyzes glycosidic bond formations from the nucleotide sugar substrate, uridine diphospho-''N''-acetyl-D-glucosamine. The nascent polysaccharide chain is then cleaved by the enzyme chitin deacetylase. The enzyme catalyzes the hydrolytic cleavage of the ''N''-acetamido group in chitin. After this the chitosan polymer chain forms micro fibrils. These fibers are embedded in an amorphous matrix consisting of proteins, glucans (which putatively cross-link the chitosan fibers), mannoproteins, lipids and other compounds.
Trisporic acid
Trisporic acid is a C-18terpenoid
The terpenoids, also known as isoprenoids, are a class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes" ...
compound that is synthesized via β-carotene
β-Carotene is an organic, strongly coloured red-orange pigment abundant in fungi, plants, and fruits. It is a member of the carotenes, which are terpenoids (isoprenoids), synthesized biochemically from eight isoprene units and thus having 40 ...
and retinol
Retinol, also called vitamin A1, is a fat-soluble vitamin in the vitamin A family found in food and used as a dietary supplement. As a supplement it is used to treat and prevent vitamin A deficiency, especially that which results in xeroph ...
pathways in the zygomycetes. It is a pheromone
A pheromone () is a secreted or excreted chemical factor that triggers a social response in members of the same species. Pheromones are chemicals capable of acting like hormones outside the body of the secreting individual, to affect the behavio ...
compound responsible for sexual differentiation in those fungal species.
History
Trisporic acid was discovered in 1964 as a metabolite that caused enhanced carotene production in ''Blakeslea trispora
''Blakeslea trispora'' is a mould and member of the division Zygomycota. This species has been well studied for its ability to produce carotenoids, particularly, β-carotene and lycopene. β-carotene is a vitamin A precursor and both of β-carot ...
''. It was later shown to be the hormone that brought about zygophore production in ''Mucor mucedo
''Mucor'' is a microbial genus of approximately 40 species of molds in the family Mucoraceae. Species are commonly found in soil, digestive systems, plant surfaces, some cheeses like Tomme de Savoie, rotten vegetable matter and iron oxide ...
''. The American mycologist and geneticist Albert Francis Blakeslee
Albert Francis Blakeslee (November 9, 1874 – November 16, 1954) was an American botanist. He is best known for his research on the poisonous jimsonweed plant and the sexuality of fungi. He was the brother of the Far East scholar George Hubbar ...
discovered that some species of Mucorales were self-sterile (heterothallic
Heterothallic species have sexes that reside in different individuals. The term is applied particularly to distinguish heterothallic fungi, which require two compatible partners to produce sexual spores, from homothallic ones, which are capable ...
), in which interactions of two strains, designated (+) and (-), are necessary for the initiation of sexual activity. This interaction was found by Hans Burgeff of the University of Goettingen to be due to the exchange of low molecular weight substances that diffused through the substratum and atmosphere. This work constituted the first demonstration of sex hormone activity in any fungus. The elucidation of the hormonal control of sexual interaction in the Mucorales extends over 60 years and involved mycologists and biochemists from Germany, Italy, the Netherlands, the UK and the USA.
Functions of trisporic acid in Mucorales
Recognition of compatible sexual partners in zygomycota is based on a cooperative biosynthesis pathway of trisporic acid. Early trisporoid derivatives and trisporic acid induce swelling of two potential hyphae, hence called zygophores, and a chemical gradient of these inducer molecules results in a growth towards each other. These progametangia come in contact with each other and build a strong connection. In the next stage, septae are established to limit the developing zygospore from the vegetative mycelium and in this way the zygophores become suspensor hyphae and gametangia are formed. After dissolving of the fusion wall, cytoplasm and a high number of nuclei from both gametangia are mixed. A selectional process (unstudied) results in a reduction of nuclei and meiosis takes place (also unstudied until today). Several cell wall modifications, as well as incorporation ofsporopollenin
270px, SEM image of pollen grains
Sporopollenin is one of the most chemically inert biological polymers. It is a major component of the tough outer (exine) walls of plant spores and pollen grains. It is chemically very stable and is usually well ...
(responsible for the dark colour of spores) take place resulting in a mature zygospore.
Trisporic acid, as the endpoint of this recognition pathway, can solely be produced in presence of both compatible partners, which enzymatically produce trisporoid precursors to be further utilized by the potential sexual partner. Species specificity of these reactions is among others obtained by spatial segregation, physicochemical features of derivatives (volatility and light sensitivity), chemical modifications of trisporoids and transcriptional/posttranscriptional regulation.

Parasexualism
Trisporoids are also used in the mediation of the recognition between parasite and host. An example is the host-parasite interaction of a parasexual nature observed between ''Parasitella parasitica'', a facultative mycoparasite of zygomycetes, and ''Absidia glauca''. This interaction is an example for biotrophic fusion parasitism, because genetic information is transferred into the host. Many morphological similarities in comparison to zygospore formation are seen, but the mature spore is called a sikyospore and is parasitic. During this process, gall-like structures are produced by the host ''Absidia glauca''. This, coupled with further evidence, has led to the assumption that trisporoids are not strictly species-specific, but that they might represent the general principle of mating recognition in Mucorales.Phototropism
Light regulation has been investigated in the zygomycetes ''Phycomyces blakesleeanus'', ''Mucor circinelloides'' and ''Pilobolus crystallinus''. For example, in ''Pilobolus crystallinus'' light is responsible for the dispersal mechanism and the sporangiophores of ''Phycomyces blakesleeanus'' grow towards light. When light, particularly blue light, is involved in the regulation of fungal development, it directs the growth of fungal structures and activates metabolic pathways. For instance, the zygomycota use light as signal to promote vegetative reproduction and growth of aerial hyphae to facilitate spore dispersal. Fungal phototropism has been investigated in detail using the fruiting body, sporangiophore, of ''Phycomyces'' as a model. ''Phycomyces'' has a complex photoreceptor system. It is able to react to different light intensities and different wavelengths. In contrast to the positive reaction to blue light, there is also a negative reaction to UV light. Reactions to red light were also observed.Activation of beta-carotene biosynthesis by light
The two genes for the enzymes phytoene desaturase (carB) and the bifunctional phytoene synthase/carotene cyclase (carRA in ''Phycomyces'', carRP in ''Mucor'') are responsible for synthesis of beta-carotene. The product of the gene crgA, which was found in Mucor suppresses the carotene formation by inhibiting the accumulation of carB and carRP mRNAs.Influence of light in sporulation and sexual development
The zygomycete ''P. blakesleeanus'' builds two types of sporangiophores, the macrophores and the microphores which differ in size. The formation of these sporangiophores work at different light fluences and therefore with specific photoreceptors. Light also regulates asexual sporulation. In ''Mucor'' the product of the crgA gene acts as an activator. In contrast, the sexual development of Phycomyces is inhibited by light because of a specialized photoreceptor system.
Gravitropism
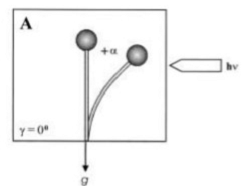
Gravitropism is a turning or growth movement by a plant or fungus in response to gravity. It is equally widespread in both kingdoms. Statolites are required in both fungi and plants for the mechanism of gravity-sensing. The Zygomycota sporangiophores originate from specialized “basal hyphae” and pass through several distinctive developmental stages until the mature asexual spores are released. In addition to the positive phototropism, the sporangiophores are directed by a negative gravitropic response into a position suitable for spore dispersal and distribution. Both responses are growth reactions i.e. the bending is caused by differential growth on the respective opposite flanks of the sporangiophore, and influence each other. The only model for the mechanism of the gravitropic reaction of ''Phycomyces'' is based on the floatability of the vacuole within the surrounding cytoplasm. The resulting asymmetric distribution of the cytoplasm is proposed to generate increased wall growth on the lower side of horizonally placed sporangiophores as in the thicker cytoplasmic layer forming there the number of vesicles secreting cell-wall material would be higher than on the upper side. Gravitropic bending starts after approximately 15 – 30 min in horizontally placed sporangiophores and continues until after, approximately 12 – 14 hours, the sporangiophore tip has recovered its original vertical position. Usually, the gravitropic response is weaker compared to the phototrophic one. However, in certain conditions, equilibrium could be established and the responses are comparable. In plants and fungi, phototropism and gravitropism interact in a complex manner. During continuous irradiation with unilateral light, the sporangiophore (fruiting body) of the zygomycete fungus, Phycomyces blakesleeanus reach a bending angle of photogravitropic equilibrium at which the gravitropic and phototropic stimuli balance each other (Fig. 1, bending angle +α, due to light irradiation).
Protein crystals involved in graviperception
In ''Phycomyces blakesleeanus'', wild type sporangiophores contain large, easily seen octahedral paracrystalline crystals with size up to 5×5×5 μm. Generally, they are found near the main vacuole in clusters consisting of more than ten crystals. They are often associated to the vacuolar transepts. Sedimentation with speed of about 100 μm/s can be observed when the sporangiophores are tilted. Sliding along during sedimentation or pulling at the vacuolar membranes and transepts serves as an inter-cellular signal to a probable cytoskeleton response, and that activates receptors located in the cell membrane. These receptors in turn trigger a chain of events which finally leads to the asymmetrical growth of the cell wall. Studies of the bending angle of wild type and mutant strain sporangiophore growth have shown that mutant strains that do not have crystals exhibit reduced gravitropic response.Lipid droplets involved in graviperception
Complex of apical lipid globules are also involved in graviperception. These lipids are clustered in cellular structures, complex of lipid globules, about 0.1mm below the very tip of the apex. (Fig. 2) The globules migrate to the columella when the sporangium is formed. In mature stage this complex is believed to act as a gravireceptor due to its floatability. Mutants that lack this lipid complex show greatly lowered gravitropic response.Phylogeny
Historically, all fungi producing a zygospore were considered to be related and placed into Zygomycota. The use of molecular phylogenetics has increasingly revealed this grouping to beparaphyletic
In taxonomy (general), taxonomy, a group is paraphyletic if it consists of the group's most recent common ancestor, last common ancestor and most of its descendants, excluding a few Monophyly, monophyletic subgroups. The group is said to be pa ...
. However, the rank (i.e., phylum or subphylum) these clades is in dispute. What follows is a phylogeny of fungi with the zygomycete subphyla derived from Spatafora et al. (2016) with both possible phylum names.
Industrial uses
Many species of zygomycetes can be used in important industrial processes. A resume of them is presented in the table.Culture conditions
 The zygomycetes are able to grow in a wide range of environments. Most of them are mesophilic (growing at 10–40 °C with an optimum 20–35 °C), but some, like ''Mucor miehei'' or ''Mucor pusillus'', are thermophilic with a minimum growth temperature of about 20 °C and maximum extending up to 60 °C. Others like ''Mucor hiemalis'' can grow at temperatures below 0 °C.
Some species of the order Mucorales are able to grow under anaerobic conditions, while most of them require aerobic conditions. Furthermore, while the majority of the zygomycetes only grow at high water activities, some of them are able to grow in salt concentrations of at least 15%. Most species of ''Mucor'' grow rapidly on agar at room temperature filling the
The zygomycetes are able to grow in a wide range of environments. Most of them are mesophilic (growing at 10–40 °C with an optimum 20–35 °C), but some, like ''Mucor miehei'' or ''Mucor pusillus'', are thermophilic with a minimum growth temperature of about 20 °C and maximum extending up to 60 °C. Others like ''Mucor hiemalis'' can grow at temperatures below 0 °C.
Some species of the order Mucorales are able to grow under anaerobic conditions, while most of them require aerobic conditions. Furthermore, while the majority of the zygomycetes only grow at high water activities, some of them are able to grow in salt concentrations of at least 15%. Most species of ''Mucor'' grow rapidly on agar at room temperature filling the Petri dish
A Petri dish (alternatively known as a Petri plate or cell-culture dish) is a shallow transparent lidded dish that biologists use to hold growth medium in which cells can be cultured,R. C. Dubey (2014): ''A Textbook Of Biotechnology For Class- ...
in 2–3 days with their coarse aerial mycelium. When incubated in liquid culture under semi-anaerobic conditions, several species grow in yeast like state. Zygospore formation may be stimulated at higher temperatures of incubation (30–40 °C).  Growth of Zygomycota in solid agar can produce low or very high fibrous colony that rapidly fills the entire Petri dish. Its color may range from pure white to shades of gray or brown. In old cultures, dark pigmented sporangia are observed. Everything depends on the species and the media used. In liquid culture, Zygomycota usually form a bland mass and do not produce spores. This is because they cannot grow aerial hyphae.
Growth of Zygomycota in solid agar can produce low or very high fibrous colony that rapidly fills the entire Petri dish. Its color may range from pure white to shades of gray or brown. In old cultures, dark pigmented sporangia are observed. Everything depends on the species and the media used. In liquid culture, Zygomycota usually form a bland mass and do not produce spores. This is because they cannot grow aerial hyphae.
Culture media
Zygomycetes grow well on most standard fungalculture medium
A growth medium or culture medium is a solid, liquid, or semi-solid designed to support the growth of a population of microorganisms or cells via the process of cell proliferation or small plants like the moss ''Physcomitrella patens''. Differe ...
such as Sabouraud dextrose agar. They can also grow on both selective and non-selective media. Minimal media, supplementary media and induction media can also be used. Most zygomycetes are sensitive to cycloheximide (actidione) and this agent should not be used in culture media.
Reproduction
A common example of a zygomycete isblack bread mold
''Rhizopus stolonifer'' is commonly known as white bread mold. It is a member of ''Zygomycota'' and considered the most important species in the genus ''Rhizopus''. It is one of the most common fungi in the world and has a global distribution al ...
(''Rhizopus stolonifer''), a member of the Mucorales
The Mucorales is the largest and best studied order of zygomycete fungi. Members of this order are sometimes called pin molds. The term mucormycosis is now preferred for infections caused by molds belonging to the order Mucorales.
Systematics ...
. It spreads over the surface of bread and other food sources, sending hyphae inward to absorb nutrients. In its asexual phase it develops bulbous black sporangia
A sporangium (; from Late Latin, ) is an enclosure in which spores are formed. It can be composed of a single cell or can be multicellular. Virtually all plants, fungi, and many other lineages form sporangia at some point in their life cyc ...
at the tips of upright hyphae, each containing hundreds of haploid spore
In biology, a spore is a unit of sexual or asexual reproduction that may be adapted for dispersal and for survival, often for extended periods of time, in unfavourable conditions. Spores form part of the life cycles of many plants, algae, f ...
s.
As in most zygomycetes, asexual reproduction is the most common form of reproduction. Sexual reproduction in ''Rhizopus stolonifer'', as in other zygomycetes, occurs when haploid hyphae of different mating type
Mating types are the microorganism equivalent to sexes in multicellular lifeforms and are thought to be the ancestor to distinct Sex, sexes. They also occur in macro-organisms such as fungi.
Definition
Mating types are the microorganism equivalent ...
s are in close proximity to each other. Growth of the gametangia commences after gametangia come in contact, and plasmogamy
Plasmogamy is a stage in the sexual reproduction of fungi, in which the protoplasm of two parent cells (usually from the mycelia) fuse without the fusion of nuclei, effectively bringing two haploid nuclei close together in the same cell. This sta ...
, or the fusion of the cytoplasm, occurs. Karyogamy
Karyogamy is the final step in the process of fusing together two haploid eukaryotic cells, and refers specifically to the fusion of the two nuclei. Before karyogamy, each haploid cell has one complete copy of the organism's genome. In order for ...
, which is the fusion of the nuclei, follows closely after. The zygosporangia are then diploid
Ploidy () is the number of complete sets of chromosomes in a cell, and hence the number of possible alleles for autosomal and pseudoautosomal genes. Sets of chromosomes refer to the number of maternal and paternal chromosome copies, respectively ...
. Zygosporangia are typically thick-walled, highly resilient to environmental hardships, and metabolically inert. When conditions improve, however, they germinate to produce a sporangium or vegetative hypha
A hypha (; ) is a long, branching, filamentous structure of a fungus, oomycete, or actinobacterium. In most fungi, hyphae are the main mode of vegetative growth, and are collectively called a mycelium.
Structure
A hypha consists of one or ...
e. Meiosis occurs during germination of the zygosporangium so the resulting spores or hyphae are haploid. Grows in warm and damp conditions.
Some zygomycetes disperse their spores in a more precise manner than simply allowing them to drift aimlessly on air currents. ''Pilobolus
''Pilobolus'' is a genus of fungi that commonly grows on herbivore dung.
Life cycle
The life cycle of ''Pilobolus'' begins with a black sporangium that has been discharged onto a plant substrate such as grass. A herbivorous animal such as a h ...
'', a fungus which grows on animal dung, bends its sporangiophores towards light with the help of a light sensitive pigment ( beta-carotene) and then "fires" them with an explosive squirt of high-pressure cytoplasm
In cell biology, the cytoplasm is all of the material within a eukaryotic cell, enclosed by the cell membrane, except for the cell nucleus. The material inside the nucleus and contained within the nuclear membrane is termed the nucleoplasm. The ...
. Sporangia can be launched as far as 2 m, placing them far away from the dung and hopefully on vegetation which will be eaten by an herbivore, eventually to be deposited with dung elsewhere. Different mechanisms for forcible spore discharge have evolved among members of the zygomycete order Entomophthorales
The Entomophthorales are an order of fungi that were previously classified in the class Zygomycetes. A new subdivision, Entomophthoromycotina, has recently been circumscribed for them.
Most species of the Entomophthorales are pathogens of ins ...
.
Evolution of conidia
The evolution of theconidium
A conidium ( ; ), sometimes termed an asexual chlamydospore or chlamydoconidium (), is an asexual, non-motile spore of a fungus. The word ''conidium'' comes from the Ancient Greek word for dust, ('). They are also called mitospores due to th ...
from the sporangiospore is the main defining difference between zygomycetes and ascomycetes
Ascomycota is a phylum of the kingdom Fungi that, together with the Basidiomycota, forms the subkingdom Dikarya. Its members are commonly known as the sac fungi or ascomycetes. It is the largest phylum of Fungi, with over 64,000 species. The defi ...
. The evolution of sporangiospores typical of zygomycetes to conidia similar to those found in ascomycetes can be modeled by a series of forms seen in zygomycetes. Many zygomycetes produce multiple sporangiospores inside a single sporangium. Some have evolved multiple small sporangiola that contain few sporangiospores. In some cases, there may be a few as three spores in each sporangiolum, and a few species have sporangiola which contain just a single spore. ''Choanephora
''Choanephora'' is a genus of Zygomycota
Zygomycota, or zygote fungi, is a former division or phylum of the kingdom Fungi. The members are now part of two phyla: the Mucoromycota and Zoopagomycota. Approximately 1060 species are known. Th ...
'', a zygomycete, has a sporangiolum that contains one spore with a sporangium wall that is visible at the base of the sporangium. This structure is similar to a conidium, which has two, fused cell walls, an inner spore wall and an outer sporangium wall.
References
External links
Zygomycota
at the Tree of Life Web Project
Zygomycetes.org
List of all Zygomycetes species from Zygomycetes database by PM Kirk in Catalogue of Life 2008
* {{Taxonbar, from=Q215384 Fungus phyla Fungi by classification