Wheland intermediate on:
[Wikipedia]
[Google]
[Amazon]
 An arenium ion in
An arenium ion in 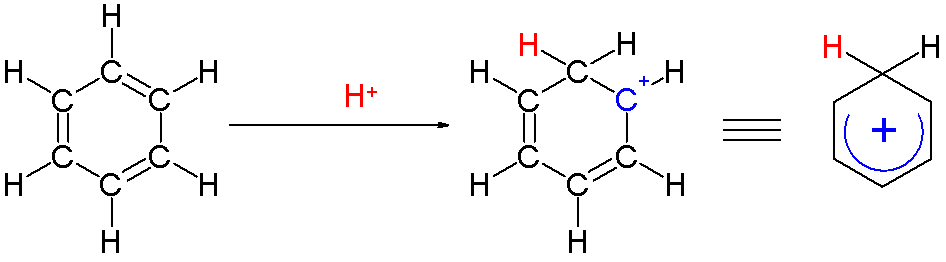 Two hydrogen atoms bonded to one carbon lie in a plane perpendicular to the benzene ring. The arenium ion is no longer an aromatic species; however it is relatively stable due to delocalization: the positive charge is delocalized over 3 carbon atoms by the
Two hydrogen atoms bonded to one carbon lie in a plane perpendicular to the benzene ring. The arenium ion is no longer an aromatic species; however it is relatively stable due to delocalization: the positive charge is delocalized over 3 carbon atoms by the  A complexed electrophile can contribute to the stability of arenium ions.
Salts of benzenium ion can be isolated when benzene is protonated by the carborane superacid H(CB11H(CH3)5Br6). The benzenium salt is crystalline with thermal stability up to 150 °C.
A complexed electrophile can contribute to the stability of arenium ions.
Salts of benzenium ion can be isolated when benzene is protonated by the carborane superacid H(CB11H(CH3)5Br6). The benzenium salt is crystalline with thermal stability up to 150 °C.  In this reaction sequence the R–Pd(II)–Br starting complex 1 stabilized by
In this reaction sequence the R–Pd(II)–Br starting complex 1 stabilized by
 An arenium ion in
An arenium ion in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
is a cyclohexadienyl cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
that appears as a reactive intermediate in electrophilic aromatic substitution
Electrophilic aromatic substitution (SEAr) is an organic reaction in which an atom that is attached to an aromatic ring, aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitut ...
.
For historic reasons this complex is also called a Wheland intermediate, after American chemist George Willard Wheland (1907–1976). They are also called sigma complexes. The smallest arenium ion is the benzenium ion (), which is protonated benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
.
: Two hydrogen atoms bonded to one carbon lie in a plane perpendicular to the benzene ring. The arenium ion is no longer an aromatic species; however it is relatively stable due to delocalization: the positive charge is delocalized over 3 carbon atoms by the
Two hydrogen atoms bonded to one carbon lie in a plane perpendicular to the benzene ring. The arenium ion is no longer an aromatic species; however it is relatively stable due to delocalization: the positive charge is delocalized over 3 carbon atoms by the pi system
In mathematics, a -system (or pi-system) on a set \Omega is a collection P of certain subsets of \Omega, such that
* P is non-empty.
* If A, B \in P then A \cap B \in P.
That is, P is a non-empty family of subsets of \Omega that is closed und ...
, as depicted on the following resonance structures:
: A complexed electrophile can contribute to the stability of arenium ions.
Salts of benzenium ion can be isolated when benzene is protonated by the carborane superacid H(CB11H(CH3)5Br6). The benzenium salt is crystalline with thermal stability up to 150 °C.
A complexed electrophile can contribute to the stability of arenium ions.
Salts of benzenium ion can be isolated when benzene is protonated by the carborane superacid H(CB11H(CH3)5Br6). The benzenium salt is crystalline with thermal stability up to 150 °C. Bond length
In molecular geometry, bond length or bond distance is defined as the average distance between Atomic nucleus, nuclei of two chemical bond, bonded atoms in a molecule. It is a Transferability (chemistry), transferable property of a bond between at ...
s deduced from X-ray crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ...
are consistent with a cyclohexadienyl cation structure.
In one study a methylene arenium ion is stabilized by metal complexation:
: In this reaction sequence the R–Pd(II)–Br starting complex 1 stabilized by
In this reaction sequence the R–Pd(II)–Br starting complex 1 stabilized by TMEDA
Tetramethylethylenediamine (TMEDA or TEMED) is a chemical compound with the formula (CH3)2NCH2CH2N(CH3)2. This species is derived from ethylenediamine by replacement of the four amine hydrogens with four methyl groups. It is a colorless liquid, ...
is converted through dppe to metal complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or ...
2. Electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carr ...
attack of methyl triflate
Methyl trifluoromethanesulfonate, also commonly called methyl triflate and abbreviated MeOTf, is the organic compound with the formula . It is a colourless liquid which finds use in organic chemistry as a powerful methylating agent. The compoun ...
forms methylene arenium ion 3 with (based on X-ray crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ...
) positive charge located in aromatic para position and with the methylene group 6° out of the plane of the ring. Reaction first with water and then with triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. Like triethanolamine and the tetraethylammonium ion, it is often abbreviated TEA. It is a colourless volatile liquid with a strong fishy odor remini ...
hydrolyzes the ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R†...
group.
See also
*Aryl radical An aryl radical in organic chemistry is a reactive intermediate and an arene compound incorporating one free radical carbon atom as part of the ring structure. As such it is the radical counterpart of the arenium ion. The parent compound is the ...
* Cyclopentadienyl anion
Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are pin ...
* Meisenheimer complex A Meisenheimer complex or Jackson–Meisenheimer complex in organic chemistry is a 1:1 reaction adduct between an arene compound, arene carrying electron withdrawing groups and a nucleophile. These complexes are found as reactive intermediates in nu ...
, the analogous intermediate in nucleophilic aromatic substitution
* Tropylium cation
The tropylium ion or cycloheptatrienyl cation is an aromatic species with a formula of 7H7sup>+. Its name derives from the molecule tropine from which cycloheptatriene (tropylidene) was first synthesized in 1881. Salts of the tropylium cation ...
Some historic references
* *References
{{Reflist Reactive intermediates Carbocations