TNP-ATP on:
[Wikipedia]
[Google]
[Amazon]
TNP-ATP is a 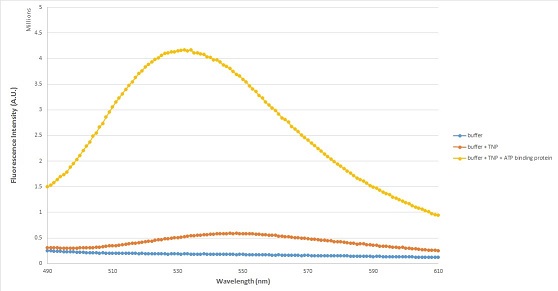
fluorescent
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
that is able to determine whether a protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
binds to ATP
ATP may refer to:
Companies and organizations
* Association of Tennis Professionals, men's professional tennis governing body
* American Technical Publishers, employee-owned publishing company
* ', a Danish pension
* Armenia Tree Project, non ...
, and the constants associated with that binding. It is primarily used in fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter wa ...
, but is also very useful as an acceptor molecule in FRET
A fret is any of the thin strips of material, usually metal wire, inserted laterally at specific positions along the neck or fretboard of a stringed instrument. Frets usually extend across the full width of the neck. On some historical instrume ...
, and as a fluorescent probe in fluorescence microscopy
Microscopy is the technical field of using microscopes to view objects and areas of objects that cannot be seen with the naked eye (objects that are not within the resolution range of the normal eye). There are three well-known branches of micr ...
and X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
. 
Constituent parts
TNP refers to thechemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
2,4,6-trinitrophenol, also known as Picric acid
Picric acid is an organic compound with the formula (O2N)3C6H2OH. Its IUPAC name is 2,4,6-trinitrophenol (TNP). The name "picric" comes from el, πικρός (''pikros''), meaning "bitter", due to its bitter taste. It is one of the most acidic ...
. It is a primary constituent of many unexploded landmines, and is a cousin to TNT, but less stable. It is recognized as an environmental contaminant and is toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subst ...
to many organisms. It is still commonly used in the manufacturing of fireworks
Fireworks are a class of Explosive, low explosive Pyrotechnics, pyrotechnic devices used for aesthetic and entertainment purposes. They are most commonly used in fireworks displays (also called a fireworks show or pyrotechnics), combining a l ...
, explosives
An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An expl ...
, and rocket fuels
Rocket propellant is the reaction mass of a rocket. This reaction mass is ejected at the highest achievable velocity from a rocket engine to produce thrust. The energy required can either come from the propellants themselves, as with a chemical ...
, as well as in leather, pharmaceutical, and dye industries.
ATP
ATP may refer to:
Companies and organizations
* Association of Tennis Professionals, men's professional tennis governing body
* American Technical Publishers, employee-owned publishing company
* ', a Danish pension
* Armenia Tree Project, non ...
is an essential mediator of life. It is used to overcome unfavorable energy barriers to initiate and fuel chemical reactions. It is also used to drive biological machinery and regulate a number of processes via protein-phosphorylation
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, wh ...
. However, the proteins that bind ATP for both regulation and enzymatic
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
reactions are very diverse—many yet undiscovered—and for many proteins their relationship to ATP in terms of number of binding sites, binding constant
The binding constant, or affinity constant/association constant, is a special case of the equilibrium constant ''K'', and is the inverse of the dissociation constant. It is associated with the binding and unbinding reaction of receptor (R) and lig ...
s, and dissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant (K_D) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex fa ...
s remain unclear.
TNP-ATP
Conjugating TNP to ATP renders this nucleotide triphosphate fluorescent and colored whilst allowing it to retain its biological activity. TNP-ATP is thus afluorescent
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
analog of ATP. This conjugation is very useful in providing information about interactions between ATP and an ATP-binding protein because TNP-ATP interacts with proteins and enzymes as a substitute for its parent nucleotide, and has a strong binding affinity for most systems that require ATP.
TNP is excited at a wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, tro ...
of 408 and 470 nm, and fluoresces in the 530–560 nm range. This is a very useful range of excitation because it is far from where proteins or nucleotides absorb. When TNP-ATP is in water or other aqueous solutions, this emission is very weak. However, once TNP-ATP binds to a protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
, there is a dramatic increase in fluorescent intensity. This property enables researchers to study various proteins’ binding interaction with ATP. Thus, with enhanced fluorescence, it can be seen whether a protein binds to ATP.
When TNP-ATP in water is excited at 410 nm, TNP-ATP shows a single fluorescence maximum at 561 nm. This maximum shifts as the fluid's viscosity changes. For example, in N,N-dimethylformamide
Dimethylformamide is an organic compound with the formula ( CH3)2NC(O)H. Commonly abbreviated as DMF (although this initialism is sometimes used for dimethylfuran, or dimethyl fumarate), this colourless liquid is miscible with water and the major ...
, instead of having its maxima at 561 nm as in water, the maxima is instead at 533 nm.
Binding to a protein will also change the wavelength of maximal emission, as well as a change in fluorescent intensity. For example, binding to the chemotaxis
Chemotaxis (from '' chemo-'' + ''taxis'') is the movement of an organism or entity in response to a chemical stimulus. Somatic cells, bacteria, and other single-cell or multicellular organisms direct their movements according to certain chemica ...
protein CheA indicates a severalfold enhancement of fluorescence intensity and a blue-shift in wavelength of the maximal emission.
Using this TNP nucleotide analog has been shown in many instances to be superior to traditional radionucleotide-labelling based techniques. The health concerns and the cost associated with the use of radioactive isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) ...
s makes TNP-ATP an attractive alternative.
The first fluorescent ribose
Ribose is a simple sugar and carbohydrate with molecular formula C5H10O5 and the linear-form composition H−(C=O)−(CHOH)4−H. The naturally-occurring form, , is a component of the ribonucleotides from which RNA is built, and so this compo ...
-modified ATP is 2’,3’-O-(2,4,7-trinitrocyclohexadienylidene) adenosine 5’triphosphate (TNP-ATP), and was introduced in 1973 by Hiratsuka and Uchida. TNP-ATP was originally synthesized to investigate the ATP binding site of myosin ATPase
Myosin ATPase () is an enzyme with systematic name ''ATP phosphohydrolase (actin-translocating)''. This enzyme catalyses the following chemical reaction
: ATP + H2O \rightleftharpoons ADP + phosphate
ATP hydrolysis provides energy for actomyosi ...
. Reports of TNP-ATP’s success in the investigation of this motor protein extended TNP-ATP’s use to other proteins and enzymes. TNP-ATP has now been used as a spectroscopic probe for numerous proteins suspected to have ATP interactions. These include several protein kinase
In biochemistry, a kinase () is an enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates. This process is known as phosphorylation, where the high-energy ATP molecule don ...
s, ATPase
ATPases (, Adenosine 5'-TriPhosphatase, adenylpyrophosphatase, ATP monophosphatase, triphosphatase, SV40 T-antigen, ATP hydrolase, complex V (mitochondrial electron transport), (Ca2+ + Mg2+)-ATPase, HCO3−-ATPase, adenosine triphosphatase) are ...
s, myosin
Myosins () are a superfamily of motor proteins best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are ATP-dependent and responsible for actin-based motility.
The first myosin ...
, and other nucleotide binding proteins. Over the past twenty years, there have been hundreds of papers describing TNP-ATP’s use and applications. Many applications involving this fluorescently labeled nucleotide have helped to clarify structure-function relationships of many ATP-requiring proteins and enzymes. There have also been a growing number of papers that display TNP-ATP use as a means of assessing the ATP-binding capacity of various mutant proteins.
Preparation
Preparing TNP-ATP is a one-step synthesis that is relatively safe and easy. Adenosine’s ribose moiety can be trinitrophenylated by 2,4,6-trinitrobenzene-1-sulfonate ( TNBS). The resulting compound assumes a bright orange color and has visible absorption characteristics, as is characteristic of a Meiseinheimer spiro complex compound linking. To see the exact method of preparion, please refer to T. Hiratsuka's and K. Uchida's paper ''"Preparation and Properties of 2'(r 3')-O(2,4,6-trinitrophenyl) Adenosine 5'-triphosphate, an Analog of Adenosine Triphosphate,"'' found in the reference section. To revert TNP-ATP back to its constituent parts, or in other words tohydrolyze
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
TNP-ATP to give equilmolar amounts of picric acid (TNP) and ATP, TNP-ATP should be treated with 1 M HCl HCL may refer to:
Science and medicine
* Hairy cell leukemia, an uncommon and slowly progressing B cell leukemia
* Harvard Cyclotron Laboratory, from 1961 to 2002, a proton accelerator used for research and development
* Hollow-cathode lamp, a spe ...
at 100 degrees Celsius for 1.5 hours. This is because if TNP-ATP is acidified under mild conditions, it results in the opening of the dioxolane
Dioxolane is a heterocyclic acetal with the chemical formula (CH2)2O2CH2. It is related to tetrahydrofuran by interchange of one oxygen for a CH2 group. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2-dioxo ...
ring attached to the 2’-oxygen, leaving a 3’O-TNP derivative as the only product.
Storage
TNP-ATP should be stored at −20 °C, in the dark, and used under minimal lighting conditions. When in solution, TNP-ATP has a shelf life of about 30 days.pKa and isosbestic point
When absorption was measured against wavelength at various pH values, the changes at wavelength 408 nm and 470 nm yielded a sigmoidal line with a midpoint at 5.1. This indicated that the absorbance at these two wavelengths depends upon the ionization of thechromophoric
A chromophore is the part of a molecule responsible for its color.
The color that is seen by our eyes is the one not absorbed by the reflecting object within a certain wavelength spectrum of visible light. The chromophore is a region in the molec ...
portion of TNP-ATP and is unaffected by ionization of ATP. Although this ionization constant of 5.1 is not in physiological range, it has been shown that the absorbance of TNP-ATP is sensitive enough to detect changes due to slight shifts in neutral pH. Spectroscopic superposition indicated TNP-ATP’s isosbestic
In spectroscopy, an isosbestic point is a specific wavelength, wavenumber or frequency at which the total absorbance of a sample does not change during a chemical reaction or a physical change of the sample. The word derives from two Greek words ...
point to be 339 nm.
Constants and calculations
At low concentrations of TNP-ATP (≤1 μM), fluorescent intensity is proportional to the concentration of TNP added. However, at concentrations exceeding 1 μM, inner filter effects cause this relationship to no longer be linear. To correct this, researchers must determine the ratio of the predicted theoretical fluorescence intensity (assuming linearity) to the observed fluorescence intensity and then apply this correction factor. However, in most cases, researchers will try to keep the concentration of TNP to lower than 1 μM. To determine binding affinities, TNP-ATP is added to a solution and then titrated with protein. This produces a saturation curve from which the binding affinity can be determined. The number of binding sites may also be determined through this saturation curve by looking to see if there are sudden changes in slope. One can alsotitrate
Titration (also known as titrimetry and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to be analyzed). A reagent, termed the ''titrant'' ...
a fixed amount of protein with increasing additions of TNP-ATP to obtain a saturation curve. To do so, however, may get complicated due to the inner filter effects that will need to be corrected for.
To determine dissociation constants, TNP-ATP can be competed off of a protein with ATP. The value of the dissociation constant Kd for a single-site binding can then be obtained by applying the Langmuir equation for a curve fit:
where RFU is relative fluorescent units, RFUobs is the fluorescence observed, RFUfree is the fluorescence of free TNP-ATP, and RFUbound is the fluorescence of TNP-ATP when completely bound to a protein.
To measure an ATP competitor, one can add competitor to pre-incubated samples of protein:TNP-ATP. The fraction of TNP-ATP bound to the protein can be calculated via:
where θ is that fraction, and RFUmax is the value of fluorescence intensity at saturation, meaning when 100% of TNP-ATP is bound.
The dissociation constants for TNP and competitor can then be calculated through the equation:
For reasons not yet fully understood, TNP-ATP typically binds the ATP binding sites of proteins and enzymes anywhere from one to three times tighter than regular ATP. The dissociation constants are usually around 0.3–50 μM.
Other uses
In addition to using TNP-ATP to determine whether a protein binds ATP, its binding affinity and dissociation constants, and number of binding sites, TNP-ATP can also be used in ligand binding studies. To do this, titrations of the protein are added to TNP-ATP. Then, ligand is added to displace the bound analog. This is measured by decreases in fluorescence. One can also do this by titrating protein with TNP-ATP in the presence and absence of varying concentrations of the ligand of interest. Using either experiment will allow the binding affinity of the ligand to protein to be measured. TNP-ATP is also valuable fluorescence acceptor. This is because, as with any good acceptor, TNP-ATP absorbs over a wide wavelength range that corresponds to the range of emission of commonFRET
A fret is any of the thin strips of material, usually metal wire, inserted laterally at specific positions along the neck or fretboard of a stringed instrument. Frets usually extend across the full width of the neck. On some historical instrume ...
donors. Thus, TNP-ATP can be used to look at the conformational changes that proteins undergo. For example, Na+/K+ ATPase, the distance between the active site and Cys457 was shown to change from 25 Angstroms to 28 Angstroms in changing from the Na+ conformation to the K+ conformation.
In addition to fluorescent spectroscopy, TNP-ATP is very useful in fluorescent microscopy
Microscopy is the technical field of using microscopes to view objects and areas of objects that cannot be seen with the naked eye (objects that are not within the resolution range of the normal eye). There are three well-known branches of micr ...
. This is because it greatly increases the sensitivity of the observations when bound to proteins—the enhanced fluorescence greatly reduces the problem of background fluorescence. This is especially true under epifluorescent illumation (illumination and light are both on the same side of the specimen).
TNP-ATP has also been used in X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
because it can be used to determine binding constants of crystallized substrates. This technique also demonstrates the structure of proteins in the presence or absence of TNP-ATP, which may or may not correspond to the structure of proteins when they bind ATP.
References
{{Reflist Biophysics Biochemistry Cell biology Physiology Nucleotides Purines Spectroscopy Fluorescence