
Synthetic cannabinoids are a class of
designer drug
A designer drug is a structural or functional analog of a controlled substance that has been designed to mimic the pharmacological effects of the original drug, while avoiding classification as illegal and/or detection in standard drug tests. Des ...
molecules that
bind
BIND () is a suite of software for interacting with the Domain Name System (DNS). Its most prominent component, named (pronounced ''name-dee'': , short for ''name daemon''), performs both of the main DNS server roles, acting as an authoritative ...
to the same receptors to which
cannabinoid
Cannabinoids () are several structural classes of compounds found in the cannabis plant primarily and most animal organisms (although insects lack such receptors) or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tet ...
s (
THC
Tetrahydrocannabinol (THC) is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term ''THC' ...
,
CBD and many others) in
cannabis
''Cannabis'' () is a genus of flowering plants in the family Cannabaceae. The number of species within the genus is disputed. Three species may be recognized: ''Cannabis sativa'', '' C. indica'', and '' C. ruderalis''. Alternatively ...
plants attach. These
novel
A novel is a relatively long work of narrative fiction, typically written in prose and published as a book. The present English word for a long work of prose fiction derives from the for "new", "news", or "short story of something new", itsel ...
psychoactive substances
A psychoactive drug, psychopharmaceutical, psychoactive agent or psychotropic drug is a chemical substance, that changes functions of the nervous system, and results in alterations in perception, mood, consciousness, cognition or behavior.
T ...
should not be confused with synthetic
phytocannabinoids
Cannabinoids () are several structural classes of compounds found in the cannabis plant primarily and most animal organisms (although insects lack such receptors) or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tet ...
(THC or CBD obtained by
chemical synthesis
As a topic of chemistry, chemical synthesis (or combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In moder ...
) or synthetic
endocannabinoids
Cannabinoids () are several structural classes of compounds found in the cannabis plant primarily and most animal organisms (although insects lack such receptors) or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tet ...
from which they are in many aspects distinct.
Typically, synthetic cannabinoids are sprayed onto plant matter
and are usually smoked,
although they have also been ingested as a concentrated liquid form in the US and UK since 2016.
They have been marketed as herbal incense, or "herbal smoking blends",
and sold under common names like K2, spice,
and synthetic marijuana.
They are often labeled "not for human consumption" for liability defense.
A large and complex variety of synthetic cannabinoids are designed in an attempt to avoid legal restrictions on
cannabis
''Cannabis'' () is a genus of flowering plants in the family Cannabaceae. The number of species within the genus is disputed. Three species may be recognized: ''Cannabis sativa'', '' C. indica'', and '' C. ruderalis''. Alternatively ...
, making synthetic cannabinoids
designer drug
A designer drug is a structural or functional analog of a controlled substance that has been designed to mimic the pharmacological effects of the original drug, while avoiding classification as illegal and/or detection in standard drug tests. Des ...
s.
Most synthetic cannabinoids are
agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the ago ...
s of the
cannabinoid receptor
Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid recepto ...
s. They have been designed to be similar to
THC
Tetrahydrocannabinol (THC) is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term ''THC' ...
, the natural
cannabinoid
Cannabinoids () are several structural classes of compounds found in the cannabis plant primarily and most animal organisms (although insects lack such receptors) or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tet ...
with the strongest binding affinity to the
CB1 receptor, which is linked to the
psychoactive
A psychoactive drug, psychopharmaceutical, psychoactive agent or psychotropic drug is a chemical substance, that changes functions of the nervous system, and results in alterations in perception, mood, consciousness, cognition or behavior.
Th ...
effects or "high" of
marijuana
Cannabis, also known as marijuana among other names, is a psychoactive drug from the cannabis plant. Native to Central or South Asia, the cannabis plant has been used as a drug for both recreational and entheogenic purposes and in various tra ...
. These synthetic analogs often have greater
binding affinity
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from ''ligare'', which means 'to bind'. In protein-ligand binding, the ligand is usually a mol ...
and greater
potency
Potency may refer to:
* Potency (pharmacology), a measure of the activity of a drug in a biological system
* Virility
* Cell potency, a measure of the differentiation potential of stem cells
* In homeopathic dilutions, potency is a measure of how ...
to the CB
1 receptors. There are several synthetic cannabinoid families (e.g.
AM-''xxx'', ,
HU-''xx'',
JWH-''xxx'')
which are classified by the creator of the substance (e.g. JWH stands for
John W. Huffman
John William Huffman (1932–2022) was a professor of organic chemistry at Clemson University who first synthesised novel cannabinoids. His research, funded by the National Institute on Drug Abuse, was focused on making a drug to target endocanna ...
), which can include several substances with different base structures such as classical cannabinoids and unrelated naphthoylindoles.
Synthetic marijuana compounds began to be manufactured and sold in the early 2000s.
From 2008 to 2014, 142 synthetic cannabinoid receptor agonists were reported to the
European Monitoring-Center for Drugs and Drug Addiction (EMCDDA).
Reported user negative effects include
palpitations
Palpitations are perceived abnormalities of the heartbeat characterized by awareness of cardiac muscle contractions in the chest, which is further characterized by the hard, fast and/or irregular beatings of the heart.
Symptoms include a rapi ...
,
paranoia
Paranoia is an instinct or thought process that is believed to be heavily influenced by anxiety or fear, often to the point of delusion and irrationality. Paranoid thinking typically includes persecutory beliefs, or beliefs of conspiracy concer ...
, intense
anxiety
Anxiety is an emotion which is characterized by an unpleasant state of inner turmoil and includes feelings of dread over anticipated events. Anxiety is different than fear in that the former is defined as the anticipation of a future threat wh ...
,
nausea
Nausea is a diffuse sensation of unease and discomfort, sometimes perceived as an urge to vomit. While not painful, it can be a debilitating symptom if prolonged and has been described as placing discomfort on the chest, abdomen, or back of the ...
, vomiting, confusion, poor coordination, and seizures. There have also been reports of a strong compulsion to re-dose,
withdrawal symptoms
Drug withdrawal, drug withdrawal syndrome, or substance withdrawal syndrome, is the group of symptoms that occur upon the abrupt discontinuation or decrease in the intake of pharmaceutical or recreational drugs.
In order for the symptoms of wit ...
, and persistent cravings.
There have been several deaths linked to synthetic cannabinoids. The
Centers for Disease Control and Prevention
The Centers for Disease Control and Prevention (CDC) is the national public health agency of the United States. It is a United States federal agency, under the Department of Health and Human Services, and is headquartered in Atlanta, Georgi ...
(CDC) found that the number of deaths from synthetic cannabinoid use tripled between 2014 and 2015.
[10. How harmful is K2/Spice (synthetic marijuana or synthetic cannabinoids)?]
/ref> In 2018, the United States Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food s ...
warned of significant health risks from synthetic cannabinoid products that contain the rat poison brodifacoum
Brodifacoum is a highly lethal 4-hydroxycoumarin vitamin K antagonist anticoagulant poison. In recent years, it has become one of the world's most widely used pesticides. It is typically used as a rodenticide, but is also used to control larger ...
, which is added because it is thought to extend the duration of the drugs' effects.
Synthetic cannabinoid products
Synthetic cannabinoids reagent testing
Reagent testing is one of the processes used to identify substances contained within a pill, usually illicit substances.
With the increased prevalence of drugs being available in their pure forms, the terms "drug checking" or "pill testing" may a ...
kits recently became economical. It is often difficult to determine what is in these products without reagent testing because masking agent
A masking agent is a reagent used in chemical analysis which reacts with chemical species that may interfere in the analysis.
In sports a masking agent is used to hide or prevent detection of a banned substance or illegal drug like anabolic stero ...
s, such as tocopherol
Tocopherols (; TCP) are a class of organic chemical compounds (more precisely, various methylated phenols), many of which have vitamin E activity. Because the vitamin activity was first identified in 1936 from a dietary fertility factor in rats, i ...
(or vitamin E acetate
A vitamin is an organic molecule (or a set of molecules closely related chemically, i.e. vitamers) that is an essential micronutrient that an organism needs in small quantities for the proper functioning of its metabolism. Essential nutrie ...
that causes vaping-associated pulmonary injury
Vaping-associated pulmonary injury (VAPI), also known as vaping-associated lung injury (VALI) or e-cigarette, or vaping, product use associated lung injury (E/VALI), is an umbrella term, used to describe lung diseases associated with the use of v ...
), eugenol
Eugenol is an allyl chain-substituted guaiacol, a member of the allylbenzene class of chemical compounds. It is a colorless to pale yellow, aromatic oily liquid extracted from certain essential oils especially from clove, nutmeg, cinnamon, basil ...
, and fatty acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, fr ...
s, are added to confound identification. Just as the synthetic used differ between each synthetic cannabinoid product sold, so do the other contents of the counterfeit product.
Counterfeit black market cannabis products
 * Counterfeit cannabis-liquid (c-liquid) for
* Counterfeit cannabis-liquid (c-liquid) for e-cigarette
An electronic cigarette is an electronic device that simulates tobacco smoking. It consists of an atomizer, a power source such as a battery, and a container such as a cartridge or tank. Instead of smoke, the user inhales vapor. As such ...
s: Synthetic cannabinoids are increasingly offered in e-cigarette form as "c-liquid". Several schoolchildren in Greater Manchester collapsed after vaping synthetic cannabinoids mis-sold as THC e-liquid.
* Counterfeit cannabis
''Cannabis'' () is a genus of flowering plants in the family Cannabaceae. The number of species within the genus is disputed. Three species may be recognized: ''Cannabis sativa'', '' C. indica'', and '' C. ruderalis''. Alternatively ...
buds: Hemp buds (or low-potency cannabis buds) laced with synthetic cannabinoids.
* Counterfeit cannabis edible
A cannabis edible, also known as a cannabis-infused food or simply an edible, is a food product (either homemade or produced commercially) that contains decarboxylated cannabinoids (cannabinoid acids converted to their orally bioactive form) f ...
: The Florida Poison Information Center in Jacksonville warned parents in September 2020 that the number of people poisoned by fake marijuana edibles and candies has tripled.
* Counterfeit hashish
Hashish ( ar, حشيش, ()), also known as hash, "dry herb, hay" is a drug made by compressing and processing parts of the cannabis plant, typically focusing on flowering buds (female flowers) containing the most trichomes. European Monitorin ...
: From December 2018, different samples of hashish
Hashish ( ar, حشيش, ()), also known as hash, "dry herb, hay" is a drug made by compressing and processing parts of the cannabis plant, typically focusing on flowering buds (female flowers) containing the most trichomes. European Monitorin ...
have been found to contain synthetic cannabinoids.
Counterfeit CBD products
Synthetic cannabinoids appear in many CBD brands in products such as gummy bears
Gummy bears (German: ''Gummibär'') are small, fruit gum candies, similar to a jelly baby in some English-speaking countries. The candy is roughly long and shaped in the form of a bear. The gummy bear is one of many gummies, popular gelat ...
and vape cartridges.
"Herb/incense" blends
Synthetic cannabinoids found in herb blends
Synthetic cannabinoid components of ‘Spice’ (a non-exhaustive list):
Non-cannabinoid chemicals found in herb blends
Most blends consist of synthetic cannabinoids sprayed onto inert vegetable matter, but some contain other psychoactive substances
A psychoactive drug, psychopharmaceutical, psychoactive agent or psychotropic drug is a chemical substance, that changes functions of the nervous system, and results in alterations in perception, mood, consciousness, cognition or behavior.
T ...
, including psychoactive herbs, e.g., "Wild Dagga" and "Indian Warrior," and psychoactive alkaloid
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar ...
s, e.g., betonicine, aporphine
Aporphine is an alkaloid with the chemical formula . The IUPAC (International Union of Pure and Applied Chemistry) name of aporphine is ''6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo e,guinoline.'' It is the core chemical substructure of the aporphine ...
, leonurine
Leonurine (also known as "SCM-198" in research) is a pseudoalkaloid that has been isolated from ''Leonotis leonurus'', ''Leonotis nepetifolia'', ''Leonurus japonicus'', '' Leonurus cardiaca'' (motherwort), '' Leonurus sibiricus'', as well as oth ...
, nuciferine
Nuciferine is an alkaloid found within the plants ''Nymphaea caerulea'' and ''Nelumbo nucifera''.
Preliminary psychopharmacological research in 1978 was unable to conclusively determine the compound's classification in regards to dopamine-recepto ...
, and nicotine
Nicotine is a natural product, naturally produced alkaloid in the nightshade family of plants (most predominantly in tobacco and ''Duboisia hopwoodii'') and is widely used recreational drug use, recreationally as a stimulant and anxiolytic. As ...
. Some synthetic cannabinoids products have also been found to contain synthetic opioids
Opioids are substances that act on opioid receptors to produce morphine-like effects. Medically they are primarily used for pain relief, including anesthesia. Other medical uses include suppression of diarrhea, replacement therapy for opioid us ...
. For example, in 2010, nine people died due to the combination of ''O''-desmethyltramadol, a μ-opioid agonist
Opioids are substances that act on opioid receptors to produce morphine-like effects. Medically they are primarily used for pain relief, including anesthesia. Other medical uses include suppression of diarrhea, replacement therapy for opioid use ...
and analgesic
An analgesic drug, also called simply an analgesic (American English), analgaesic (British English), pain reliever, or painkiller, is any member of the group of drugs used to achieve relief from pain (that is, analgesia or pain management). It ...
drug, and Kratom
''Mitragyna speciosa'' (commonly known as kratom, an herbal leaf from a tree of the Rubiaceae family, ) is a tropical evergreen tree in the coffee family native to Southeast Asia. It is indigenous to Thailand, Indonesia, Malaysia, Myanmar, and ...
, an Asiatic medicinal plant containing mitragynine
Mitragynine is an indole-based alkaloid and the most abundant active alkaloid in the Southeast Asian plant ''Mitragyna speciosa'', commonly known as '' kratom.'' The total alkaloid concentration in dried leaves ranges from 0.5 to 1.5%. In Thai va ...
, another μ-opioid agonist
Opioids are substances that act on opioid receptors to produce morphine-like effects. Medically they are primarily used for pain relief, including anesthesia. Other medical uses include suppression of diarrhea, replacement therapy for opioid use ...
, in a synthetic cannabinoid product called "Krypton."AH-7921
AH-7921 is an opioid analgesic drug selective for the μ-opioid receptor, having around 90% the potency of morphine when administered orally. It was discovered in the 1970s by a team at Allen and Hanburys located in the United Kingdom. The drug ...
was detected in smoking blends in Japan. In 2018, there was an outbreak of synthetic cannabinoids contaminated with anticoagulant
Anticoagulants, commonly known as blood thinners, are chemical substances that prevent or reduce coagulation of blood, prolonging the clotting time. Some of them occur naturally in blood-eating animals such as leeches and mosquitoes, where the ...
s, mainly brodifacoum
Brodifacoum is a highly lethal 4-hydroxycoumarin vitamin K antagonist anticoagulant poison. In recent years, it has become one of the world's most widely used pesticides. It is typically used as a rodenticide, but is also used to control larger ...
, in at least 11 states in the US that caused coagulopathy
Coagulopathy (also called a bleeding disorder) is a condition in which the blood's ability to coagulate (form clots) is impaired. This condition can cause a tendency toward prolonged or excessive bleeding (bleeding diathesis), which may occur spo ...
(prolonged or excessive bleeding) and resulted in the treatment of over 300 people and at least eight deaths.
One of the most common non-cannabinoid ingredients in these products is oleamide
Oleamide is an organic compound with the formula CH3(CH2)7CH=CH(CH2)7CONH2(. It is the amide derived from the fatty acid oleic acid. It is a colorless waxy solid and occurs in nature. Sometimes labeled as a fatty acid primary amide (FAPA), it is ...
, a fatty acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, fr ...
derivative that acts similarly to a cannabinoid
Cannabinoids () are several structural classes of compounds found in the cannabis plant primarily and most animal organisms (although insects lack such receptors) or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tet ...
and has hypnotic
Hypnotic (from Greek ''Hypnos'', sleep), or soporific drugs, commonly known as sleeping pills, are a class of (and umbrella term for) psychoactive drugs whose primary function is to induce sleep (or surgical anesthesiaWhen used in anesthesia ...
properties. Analysis of 44 products synthetic cannabinoid revealed oleamide in 7 of the products tested. Other non-cannabinoid ingredients that have been found in synthetic cannabinoid blends include harmine
Harmine is a beta-carboline and a harmala alkaloid. It occurs in a number of different plants, most notably the Syrian rue and ''Banisteriopsis caapi''. Harmine reversibly inhibits monoamine oxidase A (MAO-A), an enzyme which breaks down monoamin ...
and harmaline
Harmaline is a fluorescent indole alkaloid from the group of harmala alkaloids and beta-carbolines. It is the partly hydrogenated form of harmine.
Occurrence in nature
Various plants contain harmaline including ''Peganum harmala'' (Syrian rue) ...
, reversible monoamine oxidase inhibitor
Monoamine oxidase inhibitors (MAOIs) are a class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, espe ...
s, which have been found with myristicin
Myristicin is a naturally occurring compound found in common herbs and spices, the most well known being nutmeg. It is an insecticide, and has been shown to enhance the effectiveness of other insecticides in combination. Myristicin is also a pre ...
and asarone;substituted cathinone
Substituted cathinones, which include some stimulants and entactogens, are derivatives of cathinone. They feature a phenethylamine core with an alkyl group attached to the alpha carbon, and a ketone group attached to the beta carbon, along ...
derived stimulant drugs such as 4-methylbuphedrone and 4'-methyl-alpha-PPP; and psychedelic
Psychedelics are a subclass of hallucinogenic drugs whose primary effect is to trigger non-ordinary states of consciousness (known as psychedelic experiences or "trips").Pollan, Michael (2018). ''How to Change Your Mind: What the New Science of ...
tryptamine
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the f ...
derivatives such as 4-OH-DET.
Herbs labeled on packages marketed as legal high
Packages of synthetic cannabinoid products can claim to contain a wide array of plants. However, oftentimes, none of the listed ingredients have been detectable.
Herbal components of ‘Spice’ (a non-exhaustive list):
Naming synthetic cannabinoids
Many of the early synthetic cannabinoids that were synthesized for use in research were named after either the scientist who first synthesized them or the institution or company where they originated.
Some of the names of synthetic cannabinoids synthesized for recreational use
Recreational drug use indicates the use of one or more psychoactive drugs to induce an altered state of consciousness either for pleasure or for some other casual purpose or pastime by modifying the perceptions and emotions of the user. When a ...
were given names to help market the products. For example, AKB-48 (also known as APINACA
APINACA (AKB48, ''N''-(1-adamantyl)-1-pentyl-1''H''-indazole-3-carboxamide) is a drug that acts as a reasonably potent agonist for the cannabinoid receptors, with a Ki of 304.5nM and an EC50 of 585nM at CB1. It had never previously been report ...
) is also the name of a popular Japanese girl band; 2NE1
2NE1 (, ) was a South Korean girl group formed by YG Entertainment, which was active between 2009 and 2016. The group was composed of four members: Bom, CL, Dara, and Minzy. Known for breaking typical stereotypes of K-pop, musical experiment ...
(also known as APICA APICA or Apica may refer to:
Toponyms
* Apica River, stream in Charlevoix Regional County Municipality, Capitale-Nationale, Quebec, Canada
* Mont-Apica, Quebec, unorganized territory in Lac-Saint-Jean-Est Regional County Municipality, Saguenay� ...
) is also a South Korean girl band; and XLR-11
XLR-11 (5"-fluoro-UR-144 or 5F-UR-144) is a drug that acts as a potent agonist for the cannabinoid receptors CB1 and CB2 with EC50 values of 98 nM and 83 nM, respectively. It is a 3-(tetramethylcyclopropylmethanoyl)indole derivative related t ...
was named after the first USA-developed liquid fuel rocket for aircraft. Now many synthetic cannabinoids are assigned names derived from their four main structural components, core, tail, linker, and linked group, where the name is formatted as LinkedGroup-TailCoreLinker. For example, in 5F-MDMB-PINACA
5F-ADB (also known as 5F-MDMB-PINACA) is an indazole-based synthetic cannabinoid from the indazole-3-carboxamide family, which has been used as an active ingredient in synthetic cannabis products and has been sold online as a designer drug. 5F-A ...
(also known as 5F-ADB
5F-ADB (also known as 5F-MDMB-PINACA) is an indazole-based synthetic cannabinoid from the indazole-3-carboxamide family, which has been used as an active ingredient in synthetic cannabis products and has been sold online as a designer drug. 5F-A ...
), ''5F'' stands for the terminal fluorine or "fluorine on carbon 5" of the pentyl chain; ''MDMB'' stands for "methyl-3,3-dimethyl butanoate", the linked group; and ''PINACA'' stands for "pentyl chain (tail) indazole (core) carboxamide (linker)".
Common names
Use of the term "synthetic marijuana" to describe products containing synthetic cannabinoids is controversial and, according to Lewis Nelson, a medical toxicologist at the NYU School of Medicine, a mistake. Nelson claims that relative to marijuana
Cannabis, also known as marijuana among other names, is a psychoactive drug from the cannabis plant. Native to Central or South Asia, the cannabis plant has been used as a drug for both recreational and entheogenic purposes and in various tra ...
, products containing synthetic cannabinoids "are really quite different, and the effects are much more unpredictable. It's dangerous". Since the term ''synthetic'' does not apply to the plant, but rather to the cannabinoid
Cannabinoids () are several structural classes of compounds found in the cannabis plant primarily and most animal organisms (although insects lack such receptors) or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tet ...
that the plant
Plants are predominantly photosynthetic eukaryotes of the kingdom Plantae. Historically, the plant kingdom encompassed all living things that were not animals, and included algae and fungi; however, all current definitions of Plantae exclud ...
contains (THC
Tetrahydrocannabinol (THC) is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term ''THC' ...
), the term ''synthetic cannabinoid'' is more appropriate.
Nearly 700 "herbal incense" blends exist. They are often called "synthetic marijuana", "natural herbs", "herbal incense", or "herbal smoking blends" and often labeled "not for human consumption".Financial Times
The ''Financial Times'' (''FT'') is a British daily newspaper printed in broadsheet and published digitally that focuses on business and economic current affairs. Based in London, England, the paper is owned by a Japanese holding company, Nik ...
'', the assets of the Psyche Deli rose from £65,000 in 2006 to £899,000 in 2007. The EMCDDA
The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) is an agency of the European Union located in Lisbon, Portugal, and established in 1993. In June 2022, the Council of the European Union approved a reform of the organization ...
reported in 2009 that Spice products were identified in 21 of the 30 participating countries.
Neocannabinoids
Because of these controversies, and in particular the difficulty to distinguish natural cannabinoids obtained in laboratory (for example, CBD or synthetic THC
Tetrahydrocannabinol (THC) is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term ''THC' ...
) from artificial novel synthetic cannabinoid analog compounds not present in nature (like nabilone, Spice, the HU, JWH series, etc.), the term "neocannabinoid" has been proposed to name the latter.
Uses
Synthetic cannabinoids were made for cannabinoid
Cannabinoids () are several structural classes of compounds found in the cannabis plant primarily and most animal organisms (although insects lack such receptors) or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tet ...
research focusing on tetrahydrocannabinol
Tetrahydrocannabinol (THC) is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term ''THC'' ...
(THC), cannabinoid receptors
Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid recepto ...
, and the endocannabinoids
Cannabinoids () are several structural classes of compounds found in the cannabis plant primarily and most animal organisms (although insects lack such receptors) or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tet ...
that activate them in the body. Synthetic cannabinoids were needed partly due to legal restrictions on natural cannabinoid
Cannabinoids () are several structural classes of compounds found in the cannabis plant primarily and most animal organisms (although insects lack such receptors) or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tet ...
s, which make them difficult to obtain for research. Many have been useful because they bind selectively to either the CB1 or CB2 receptors, whereas THC
Tetrahydrocannabinol (THC) is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term ''THC' ...
has a similar affinity for both. Tritium
Tritium ( or , ) or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with half-life about 12 years. The nucleus of tritium (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus o ...
-labelled cannabinoid
Cannabinoids () are several structural classes of compounds found in the cannabis plant primarily and most animal organisms (although insects lack such receptors) or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tet ...
s such as CP-55,940
55,940 is a synthetic cannabinoid which mimics the effects of naturally occurring THC (one of the psychoactive compounds found in cannabis). CP 55,940 was created by Pfizer in 1974 but was never marketed. It is currently used to study the endoc ...
were instrumental in discovering the cannabinoid receptor
Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid recepto ...
s in the early 1990s.
Some early synthetic cannabinoids were also used clinically. Nabilone
Nabilone, sold under the brand name Cesamet among others, is a synthetic cannabinoid with therapeutic use as an antiemetic and as an adjunct analgesic for neuropathic pain. It mimics tetrahydrocannabinol (THC), the primary psychoactive compound ...
, a first generation synthetic THC analog, has been used as an antiemetic
An antiemetic is a drug that is effective against vomiting and nausea. Antiemetics are typically used to treat motion sickness and the side effects of opioid analgesics, general anaesthetics, and chemotherapy directed against cancer. They may ...
to combat vomiting and nausea since 1981. Synthetic THC (marinol, dronabinol
The International Nonproprietary Name Dronabinol, also known as delta-9-tetrahydrocannabinol, or under the trade names Marinol, Syndros, Reduvo and Adversa, is a generic name for the molecule of delta-9-tetrahydrocannabinol in the pharmaceutica ...
) has been used as an antiemetic
An antiemetic is a drug that is effective against vomiting and nausea. Antiemetics are typically used to treat motion sickness and the side effects of opioid analgesics, general anaesthetics, and chemotherapy directed against cancer. They may ...
since 1985, and an appetite stimulant since 1991, although synthetic THC is often not listed among the "synthetic cannabinoids" but as a "synthetic phytocannabinoid."recreational drug use
Recreational drug use indicates the use of one or more psychoactive drugs to induce an altered state of consciousness either for pleasure or for some other casual purpose or pastime by modifying the perceptions and emotions of the user. When a ...
in an attempt to get similar effects to cannabis. Because synthetic cannabinoid molecular structures differ from THC
Tetrahydrocannabinol (THC) is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term ''THC' ...
and other illegal cannabinoids, synthetic cannabinoids were not technically illegal. Since the discovery of the use of synthetic cannabinoids for recreational use in 2008, some synthetic cannabinoids have been made illegal, but new analogs are continually synthesized to avoid the restrictions. Synthetic cannabinoids have also been used recreationally because they are inexpensive and are typically not revealed by the standard marijuana drug tests. Unlike nabilone
Nabilone, sold under the brand name Cesamet among others, is a synthetic cannabinoid with therapeutic use as an antiemetic and as an adjunct analgesic for neuropathic pain. It mimics tetrahydrocannabinol (THC), the primary psychoactive compound ...
, the synthetic cannabinoids found being used for recreational use did not have any documented therapeutic effect
Therapeutic effect refers to the response(s) after a treatment of any kind, the results of which are judged to be useful or favorable. This is true whether the result was expected, unexpected, or even an unintended consequence. An adverse effect (i ...
s.
Toxicity
Because they activate the cannabinoid
Cannabinoids () are several structural classes of compounds found in the cannabis plant primarily and most animal organisms (although insects lack such receptors) or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tet ...
CB1 and CB2 receptors, many of the effects of synthetic cannabinoids are similar to those of THC. These are achieved at lower doses, because many synthetic cannabinoids are more potent than marijuana, and users are often unaware of exactly what they are getting and how potent it is. For example, Δ9-THC has an EC50 of 250 nM at CB1 and 1157 nM at CB2, whereas PB-22
PB-22 (QUPIC, SGT-21 or 1-pentyl-1''H''-indole-3-carboxylic acid 8-quinolinyl ester) is a designer drug offered by online vendors as a cannabimimetic agent, and detected being sold in synthetic cannabis products in Japan in 2013. PB-22 represent ...
has an EC50 of 5.1 nM at CB1 and 37 nM at CB2.Adverse effects
An adverse effect is an undesired harmful effect resulting from a medication or other intervention, such as surgery. An adverse effect may be termed a "side effect", when judged to be secondary to a main or therapeutic effect. The term complica ...
observed due to synthetic cannabinoid use include acute kidney injury
Acute kidney injury (AKI), previously called acute renal failure (ARF), is a sudden decrease in kidney function that develops within 7 days, as shown by an increase in serum creatinine or a decrease in urine output, or both.
Causes of AKI are cla ...
, cardiac toxicity
Cardiotoxicity is the occurrence of heart dysfunction as electric or muscle damage, resulting in heart toxicity. The heart becomes weaker and is not as efficient in pumping blood. Cardiotoxicity may be caused by chemotherapy (a usual example is th ...
, seizure
An epileptic seizure, informally known as a seizure, is a period of symptoms due to abnormally excessive or synchronous neuronal activity in the brain. Outward effects vary from uncontrolled shaking movements involving much of the body with los ...
, stroke
A stroke is a medical condition in which poor blood flow to the brain causes cell death. There are two main types of stroke: ischemic, due to lack of blood flow, and hemorrhagic, due to bleeding. Both cause parts of the brain to stop functionin ...
, tremor
A tremor is an involuntary, somewhat rhythmic, muscle contraction and relaxation involving oscillations or twitching movements of one or more body parts. It is the most common of all involuntary movements and can affect the hands, arms, eyes, fa ...
, hypokalemia
Hypokalemia is a low level of potassium (K+) in the blood serum. Mild low potassium does not typically cause symptoms. Symptoms may include feeling tired, leg cramps, weakness, and constipation. Low potassium also increases the risk of an abno ...
, and rhabdomyolysis
Rhabdomyolysis (also called rhabdo) is a condition in which damaged skeletal muscle breaks down rapidly. Symptoms may include muscle pains, weakness, vomiting, and confusion. There may be tea-colored urine or an irregular heartbeat. Some of th ...
.nausea
Nausea is a diffuse sensation of unease and discomfort, sometimes perceived as an urge to vomit. While not painful, it can be a debilitating symptom if prolonged and has been described as placing discomfort on the chest, abdomen, or back of the ...
, vomiting
Vomiting (also known as emesis and throwing up) is the involuntary, forceful expulsion of the contents of one's stomach through the mouth and sometimes the Human nose, nose.
Vomiting can be the result of ailments like Food-poisoning, foo ...
, confusion
In medicine, confusion is the quality or state of being bewildered or unclear. The term "acute mental confusion" , poor coordination, anxiety
Anxiety is an emotion which is characterized by an unpleasant state of inner turmoil and includes feelings of dread over anticipated events. Anxiety is different than fear in that the former is defined as the anticipation of a future threat wh ...
, and seizures. Some of the negative effects of 5F-AKB-48 reported by users included palpitations
Palpitations are perceived abnormalities of the heartbeat characterized by awareness of cardiac muscle contractions in the chest, which is further characterized by the hard, fast and/or irregular beatings of the heart.
Symptoms include a rapi ...
, paranoia
Paranoia is an instinct or thought process that is believed to be heavily influenced by anxiety or fear, often to the point of delusion and irrationality. Paranoid thinking typically includes persecutory beliefs, or beliefs of conspiracy concer ...
, intense anxiety, and a taste like burned plastic.overdose
A drug overdose (overdose or OD) is the ingestion or application of a drug or other substance in quantities much greater than are recommended. cases linked to marijuana
Cannabis, also known as marijuana among other names, is a psychoactive drug from the cannabis plant. Native to Central or South Asia, the cannabis plant has been used as a drug for both recreational and entheogenic purposes and in various tra ...
, there are deaths linked to synthetic cannabinoids each year.suicide
Suicide is the act of intentionally causing one's own death. Mental disorders (including depression, bipolar disorder, schizophrenia, personality disorders, anxiety disorders), physical disorders (such as chronic fatigue syndrome), and s ...
, falling from a height, and wandering into traffic; cardiovascular
The blood circulatory system is a system of organs that includes the heart, blood vessels, and blood which is circulated throughout the entire body of a human or other vertebrate. It includes the cardiovascular system, or vascular system, tha ...
effects; and central nervous system depression
Central nervous system (CNS) depression is a physiological state that can result in a decreased rate of breathing, decreased heart rate, and loss of consciousness possibly leading to coma or death.
It is the result of inhibited or suppressed brai ...
.
Researchers have pointed out a few ways that synthetic cannabinoids differ from marijuana, and therefore may be more dangerous. First, they often have greater intrinsic activity
Intrinsic activity (IA) and efficacy refer to the relative ability of a drug-receptor complex to produce a maximum functional response. This must be distinguished from the affinity, which is a measure of the ability of the drug to bind to its mol ...
. Many of the synthetic cannabinoids are full agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the ago ...
s of the cannabinoids receptors, CB1 and CB2, compared to THC
Tetrahydrocannabinol (THC) is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term ''THC' ...
, which is only a partial agonist
In pharmacology, partial agonists are drugs that bind to and activate a given receptor, but have only partial efficacy at the receptor relative to a full agonist. They may also be considered ligands which display both agonistic and antagonistic e ...
. Secondly, they may have other actions in the body, in addition to activating cannabinoid receptors. Some may work on NMDA
''N''-methyl--aspartic acid or ''N''-methyl--aspartate (NMDA) is an amino acid derivative that acts as a specific agonist at the NMDA receptor mimicking the action of glutamate, the neurotransmitter which normally acts at that receptor. Unlike ...
glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can syn ...
receptors.serotonin
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vas ...
, either indirectly by inhibiting MAO and increasing 5-HT1A receptor
The serotonin 1A receptor (or 5-HT1A receptor) is a subtype of serotonin receptor, or 5-HT receptor, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protei ...
expression
Expression may refer to:
Linguistics
* Expression (linguistics), a word, phrase, or sentence
* Fixed expression, a form of words with a specific meaning
* Idiom, a type of fixed expression
* Metaphorical expression, a particular word, phrase, o ...
, or by directly binding to serotonin receptors, including the 5-HT1A and 5-HT3indole
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environmen ...
moiety
Moiety may refer to:
Chemistry
* Moiety (chemistry), a part or functional group of a molecule
** Moiety conservation, conservation of a subgroup in a chemical species
Anthropology
* Moiety (kinship), either of two groups into which a society is ...
that some synthetic cannabinoids possess is similar to the structure of serotonin
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vas ...
. Third, synthetic cannabinoids may break down into metabolites, or create other by-products when heated, that may differ from marijuana. Phase 1 metabolism
Drug metabolism is the metabolism, metabolic breakdown of drugs by living organisms, usually through specialized enzyme, enzymatic systems. More generally, xenobiotic metabolism (from the Greek xenos (Greek), xenos "stranger" and biotic "related ...
of JWH-018
JWH-018 (1-pentyl-3-(1-naphthoyl)indole, NA-PIMO or AM-678) is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 cannabinoid receptors, with some selectivity for CB2. It produces effects in ...
results in at least nine monohydroxylated metabolite
In biochemistry, a metabolite is an intermediate or end product of metabolism.
The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, c ...
s, three of which have been shown to be full agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the ago ...
s of the CB1 receptors, compared to the metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cell ...
of THC
Tetrahydrocannabinol (THC) is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term ''THC' ...
, which only results in one psychoactive
A psychoactive drug, psychopharmaceutical, psychoactive agent or psychotropic drug is a chemical substance, that changes functions of the nervous system, and results in alterations in perception, mood, consciousness, cognition or behavior.
Th ...
monohydroxylated metabolite
In biochemistry, a metabolite is an intermediate or end product of metabolism.
The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, c ...
. The metabolite N-(3-hydroxypentyl) JWH-018 was found to have toxic effects that its parent compound does not.antagonists
An antagonist is a character in a story who is presented as the chief foe of the protagonist.
Etymology
The English word antagonist comes from the Greek ἀνταγωνιστής – ''antagonistēs'', "opponent, competitor, villain, enemy, riv ...
. Lastly, they may contain unwanted substances, be mislabeled, or contain different doses than advertised (in one analysis, a difference of one log unit was found).toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subst ...
compounds); however, user reports and the effects experienced by patients seeking medical care after taking synthetic cannabinoids have been published. Each of the many different synthetic cannabinoids can have different effects at different dosages. The CDC
The Centers for Disease Control and Prevention (CDC) is the national public health agency of the United States. It is a United States federal agency, under the Department of Health and Human Services, and is headquartered in Atlanta, Georgi ...
described synthetic cannabinoid overdoses between 2010 and 2015 and of 277 drug overdose patients who reported synthetic cannabinoid as the sole agent, 66.1% reported problems in the central nervous system
The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain and spinal cord. The CNS is so named because the brain integrates the received information and coordinates and influences the activity of all par ...
(e.g., agitation, coma
A coma is a deep state of prolonged unconsciousness in which a person cannot be awakened, fails to respond normally to painful stimuli, light, or sound, lacks a normal wake-sleep cycle and does not initiate voluntary actions. Coma patients exhi ...
, toxic psychosis
Substance-induced psychosis (commonly known as toxic psychosis or drug-induced psychosis) is a form of psychosis that is attributed to substance use. It is a psychosis that results from the effects of chemicals or drugs, including those produced b ...
), 17% reported cardiovascular
The blood circulatory system is a system of organs that includes the heart, blood vessels, and blood which is circulated throughout the entire body of a human or other vertebrate. It includes the cardiovascular system, or vascular system, tha ...
problems (e.g., tachycardia
Tachycardia, also called tachyarrhythmia, is a heart rate that exceeds the normal resting rate. In general, a resting heart rate over 100 beats per minute is accepted as tachycardia in adults. Heart rates above the resting rate may be normal (su ...
, bradycardia
Bradycardia (also sinus bradycardia) is a slow resting heart rate, commonly under 60 beats per minute (BPM) as determined by an electrocardiogram. It is considered to be a normal heart rate during sleep, in young and healthy or elderly adults, a ...
), 7.6% reported pulmonary
The lungs are the primary organs of the respiratory system in humans and most other animals, including some snails and a small number of fish. In mammals and most other vertebrates, two lungs are located near the backbone on either side of th ...
problems (5.4% of which had respiratory depression
Hypoventilation (also known as respiratory depression) occurs when ventilation is inadequate (''hypo'' meaning "below") to perform needed respiratory gas exchange. By definition it causes an increased concentration of carbon dioxide (hypercapnia ...
), and 4% reported acute kidney injury
Acute kidney injury (AKI), previously called acute renal failure (ARF), is a sudden decrease in kidney function that develops within 7 days, as shown by an increase in serum creatinine or a decrease in urine output, or both.
Causes of AKI are cla ...
.
Four postmortem cases linked to the synthetic cannabinoids 5F-PB-22 have been reviewed. The postmortem
An autopsy (post-mortem examination, obduction, necropsy, or autopsia cadaverum) is a surgical procedure that consists of a thorough examination of a corpse by dissection to determine the cause, mode, and manner of death or to evaluate any di ...
blood specimens contained a range of 1.1-1.5 ng/mL of 5F-PB-22. Three of the four cases were sudden episodes and the symptoms leading to death included acute shortness of breath; vasocongestion
Vasocongestion, vascular congestion or vascular engorgement is the swelling of bodily tissues caused by increased vascular blood flow and a localized increase in blood pressure. Typical causes of vasocongestion in humans includes menstruation, sexu ...
in the liver, spleen, and kidneys; bilateral pulmonary edema
Pulmonary edema, also known as pulmonary congestion, is excessive edema, liquid accumulation in the parenchyma, tissue and pulmonary alveolus, air spaces (usually alveoli) of the lungs. It leads to impaired gas exchange and may cause hypoxemia an ...
; dead inflamed tissue (necrotizing granulomatous inflammation); and congestion of most internal organs. The fourth case presented to the hospital with severe problems that deteriorated over the course of a day, ending with circulatory
The blood circulatory system is a system of organs that includes the heart, blood vessels, and blood which is circulated throughout the entire body of a human or other vertebrate. It includes the cardiovascular system, or vascular system, tha ...
, respiratory
The respiratory system (also respiratory apparatus, ventilatory system) is a biological system consisting of specific organs and structures used for gas exchange in animals and plants. The anatomy and physiology that make this happen varies grea ...
, central nervous system
The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain and spinal cord. The CNS is so named because the brain integrates the received information and coordinates and influences the activity of all par ...
, and renal failure
Kidney failure, also known as end-stage kidney disease, is a medical condition in which the kidneys can no longer adequately filter waste products from the blood, functioning at less than 15% of normal levels. Kidney failure is classified as eit ...
.
Addiction
There have been reports of a strong compulsion to re-dose, withdrawal symptoms
Drug withdrawal, drug withdrawal syndrome, or substance withdrawal syndrome, is the group of symptoms that occur upon the abrupt discontinuation or decrease in the intake of pharmaceutical or recreational drugs.
In order for the symptoms of wit ...
, and persistent cravings lasting up to a week after taking synthetic cannabinoids, indicating that synthetic cannabinoids may be more addictive
Addiction is a neuropsychological disorder characterized by a persistent and intense urge to engage in certain behaviors, one of which is the usage of a drug, despite substantial harm and other negative consequences. Repetitive drug use oft ...
than marijuana
Cannabis, also known as marijuana among other names, is a psychoactive drug from the cannabis plant. Native to Central or South Asia, the cannabis plant has been used as a drug for both recreational and entheogenic purposes and in various tra ...
.
Psychosis
Studies are currently available that suggest an association between synthetic cannabinoids and psychosis
Psychosis is a condition of the mind that results in difficulties determining what is real and what is not real. Symptoms may include delusions and hallucinations, among other features. Additional symptoms are incoherent speech and behavior ...
. The use of synthetic cannabinoids can be associated with psychosis
Psychosis is a condition of the mind that results in difficulties determining what is real and what is not real. Symptoms may include delusions and hallucinations, among other features. Additional symptoms are incoherent speech and behavior ...
and physicians are beginning to investigate if some patients with inexplicable psychotic symptoms may have at one point used synthetic cannabinoids. In contrast to most other recreational drugs
Recreation is an activity of leisure, leisure being discretionary time. The "need to do something for recreation" is an essential element of human biology and psychology. Recreational activities are often done for enjoyment, amusement, or pleasur ...
, the dramatic psychotic state induced by use of synthetic cannabinoids has been reported, in multiple cases, to persist for several weeks, and in one case for seven months, after complete cessation of drug use. Some studies suggest that not only can synthetic cannabinoids induce psychosis
Psychosis is a condition of the mind that results in difficulties determining what is real and what is not real. Symptoms may include delusions and hallucinations, among other features. Additional symptoms are incoherent speech and behavior ...
, but they can worsen previously stable psychotic disorders
Psychosis is a condition of the mind that results in difficulties determining what is real and what is not real. Symptoms may include delusions and hallucinations, among other features. Additional symptoms are incoherent speech and behavior t ...
and might trigger a chronic (long-term) psychotic disorder
Psychosis is a condition of the mind that results in difficulties determining what is real and what is not real. Symptoms may include delusions and hallucinations, among other features. Additional symptoms are incoherent speech and behavior t ...
among vulnerable individuals such as those with a family history of mental illness
A mental disorder, also referred to as a mental illness or psychiatric disorder, is a behavioral or mental pattern that causes significant distress or impairment of personal functioning. Such features may be persistent, relapsing and remitti ...
. Individuals with risk factor
In epidemiology, a risk factor or determinant is a variable associated with an increased risk of disease or infection.
Due to a lack of harmonization across disciplines, determinant, in its more widely accepted scientific meaning, is often use ...
s for psychotic disorders
Psychosis is a condition of the mind that results in difficulties determining what is real and what is not real. Symptoms may include delusions and hallucinations, among other features. Additional symptoms are incoherent speech and behavior t ...
are often counseled against using synthetic cannabinoids. Psychiatrists have suggested that the lack of an antipsychotic
Antipsychotics, also known as neuroleptics, are a class of Psychiatric medication, psychotropic medication primarily used to manage psychosis (including delusions, hallucinations, paranoia or disordered thought), principally in schizophrenia but ...
chemical, like CBD in natural cannabis
''Cannabis'' () is a genus of flowering plants in the family Cannabaceae. The number of species within the genus is disputed. Three species may be recognized: ''Cannabis sativa'', '' C. indica'', and '' C. ruderalis''. Alternatively ...
, may make synthetic cannabinoids more likely to induce psychosis
Psychosis is a condition of the mind that results in difficulties determining what is real and what is not real. Symptoms may include delusions and hallucinations, among other features. Additional symptoms are incoherent speech and behavior ...
than natural cannabis
''Cannabis'' () is a genus of flowering plants in the family Cannabaceae. The number of species within the genus is disputed. Three species may be recognized: ''Cannabis sativa'', '' C. indica'', and '' C. ruderalis''. Alternatively ...
.
Structural classifications
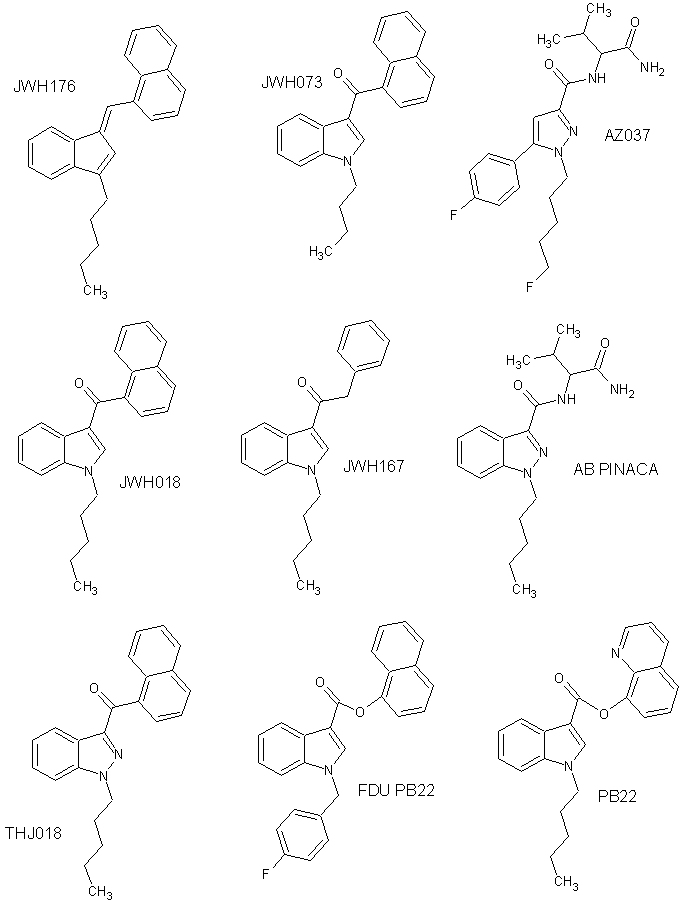
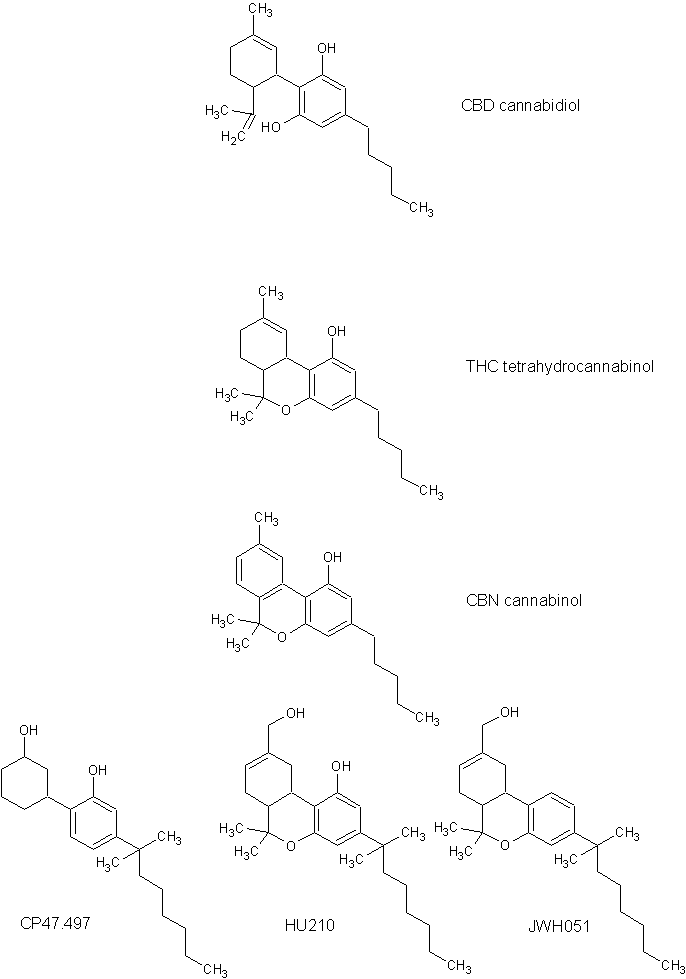 There are five major categories for synthetic cannabinoids: classical cannabinoids, non-classical cannabinoids, hybrid cannabinoids,
There are five major categories for synthetic cannabinoids: classical cannabinoids, non-classical cannabinoids, hybrid cannabinoids, aminoalkylindole Aminoalkylindoles (AAIs) are a family of cannabinergic compound that act as a cannabinoid receptor agonist. They were invented by pharmaceutical company Sterling-Winthrop in the early 1990s as potential nonsteroidal anti-inflammatory agents.
Leg ...
s, and eicosanoid
Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, around 20 carbon units in length. Eicosanoids are a s ...
s. Classical cannabinoids are analogs of THC
Tetrahydrocannabinol (THC) is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term ''THC' ...
that are based on a dibenzopyran ring. They were developed starting in the 1960s, following the isolation of THC,nabilone
Nabilone, sold under the brand name Cesamet among others, is a synthetic cannabinoid with therapeutic use as an antiemetic and as an adjunct analgesic for neuropathic pain. It mimics tetrahydrocannabinol (THC), the primary psychoactive compound ...
and dronabinol
The International Nonproprietary Name Dronabinol, also known as delta-9-tetrahydrocannabinol, or under the trade names Marinol, Syndros, Reduvo and Adversa, is a generic name for the molecule of delta-9-tetrahydrocannabinol in the pharmaceutica ...
, and one of the best known synthetic classical cannabinoids is HU-210
HU-210 is a synthetic cannabinoid that was first synthesized in 1988 from (1R,5S)-myrtenol by a group led by Raphael Mechoulam at the Hebrew University. HU-210 is 100 to 800 times more potent than natural THC from cannabis and has an extended d ...
.HU-210
HU-210 is a synthetic cannabinoid that was first synthesized in 1988 from (1R,5S)-myrtenol by a group led by Raphael Mechoulam at the Hebrew University. HU-210 is 100 to 800 times more potent than natural THC from cannabis and has an extended d ...
is a chiral compound first synthesized by Raphael Mechoulam
Raphael Mechoulam ( he, רפאל משולם, bg, Рафаел Мешулам; born 5 November 1930) is an Israeli organic chemist and professor of Medicinal Chemistry at the Hebrew University of Jerusalem in Israel. Mechoulam is best known for h ...
at Hebrew University
The Hebrew University of Jerusalem (HUJI; he, הַאוּנִיבֶרְסִיטָה הַעִבְרִית בִּירוּשָׁלַיִם) is a public research university based in Jerusalem, Israel. Co-founded by Albert Einstein and Dr. Chaim Weiz ...
in the 1980s. It was discovered in herbal incense products by the U.S. Customs and Border Protection
United States Customs and Border Protection (CBP) is the largest federal law enforcement agency of the United States Department of Homeland Security. It is the country's primary border control organization, charged with regulating and facilit ...
in January 2009; however, classical cannabinoids are not often seen in synthetic cannabinoid blends for recreational use
Recreational drug use indicates the use of one or more psychoactive drugs to induce an altered state of consciousness either for pleasure or for some other casual purpose or pastime by modifying the perceptions and emotions of the user. When a ...
, likely because they are difficult to synthesize.Pfizer
Pfizer Inc. ( ) is an American multinational pharmaceutical and biotechnology corporation headquartered on 42nd Street in Manhattan, New York City. The company was established in 1849 in New York by two German entrepreneurs, Charles Pfizer ...
as potential analgesic
An analgesic drug, also called simply an analgesic (American English), analgaesic (British English), pain reliever, or painkiller, is any member of the group of drugs used to achieve relief from pain (that is, analgesia or pain management). It ...
s.CP-47,497
CP 47,497 or (C7)-CP 47,497 is a cannabinoid receptor agonist drug, developed by Pfizer in the 1980s. It has analgesic effects and is used in scientific research. It is a potent CB1 agonist with a ''K''d of 2.1 nM.
Homologue
On the 19th of ...
( CP-47,497-C8) was one of the first synthetic cannabinoids being used recreationally. CP-47,497-C8 is made by extending the dimethylheptyl side chain of CP-47,497
CP 47,497 or (C7)-CP 47,497 is a cannabinoid receptor agonist drug, developed by Pfizer in the 1980s. It has analgesic effects and is used in scientific research. It is a potent CB1 agonist with a ''K''d of 2.1 nM.
Homologue
On the 19th of ...
to a dimethyloctyl side chain. It was discovered by forensic scientists in an herbal blend known as "Spice" in 2008, along with JWH-018
JWH-018 (1-pentyl-3-(1-naphthoyl)indole, NA-PIMO or AM-678) is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 cannabinoid receptors, with some selectivity for CB2. It produces effects in ...
, an aminoalkylindole Aminoalkylindoles (AAIs) are a family of cannabinergic compound that act as a cannabinoid receptor agonist. They were invented by pharmaceutical company Sterling-Winthrop in the early 1990s as potential nonsteroidal anti-inflammatory agents.
Leg ...
.AM-4030
AM-4030 is an analgesic drug which is a cannabinoid receptor agonist. It is a derivative of HU-210 which has been substituted with a 6β-((E)-3-hydroxyprop-1-enyl) group. This adds a "southern" aliphatic hydroxyl group to the molecule as seen in ...
, a derivative of HU-210
HU-210 is a synthetic cannabinoid that was first synthesized in 1988 from (1R,5S)-myrtenol by a group led by Raphael Mechoulam at the Hebrew University. HU-210 is 100 to 800 times more potent than natural THC from cannabis and has an extended d ...
, is a hybrid cannabinoid because it has the dibenzopyran ring common of classical cannabinoids and an aliphatic hydroxyl group common in the CP family of nonclassical cannabinoids.
Aminoalkylindole Aminoalkylindoles (AAIs) are a family of cannabinergic compound that act as a cannabinoid receptor agonist. They were invented by pharmaceutical company Sterling-Winthrop in the early 1990s as potential nonsteroidal anti-inflammatory agents.
Leg ...
s are structurally dissimilar to THC and include naphthoylindole
Naphthoylindoles are a class of synthetic cannabinoids.
See also
* Structural scheduling of synthetic cannabinoids To combat the illicit synthetic cannabinoid industry many jurisdictions have created a system to control these cannabinoids through ...
s (JWH-018
JWH-018 (1-pentyl-3-(1-naphthoyl)indole, NA-PIMO or AM-678) is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 cannabinoid receptors, with some selectivity for CB2. It produces effects in ...
), phenylacetylindole
Phenylacetylindoles are a class of synthetic cannabinoids.
In the United States, all CB1 receptor agonists of the 3-phenylacetylindole class are Schedule I Controlled Substance
This is the list of Schedule I drugs as defined by the United State ...
s (JWH-250
JWH-250 or (1-pentyl-3-(2-methoxyphenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family that acts as a cannabinoid agonist at both the CB1 and CB2 receptors, with a ''K''i of 11 nM at CB1 and 33 nM at CB2. U ...
), and benzoylindoles (AM-2233
AM-2233 is a drug that acts as a highly potent full agonist for the cannabinoid receptors, with a ''K''i of 1.8 nM at CB1 and 2.2 nM at CB2 as the active (''R'') enantiomer. It was developed as a selective radioligand for the cannab ...
). Aminoalkylindoles are considered to be the most common synthetic cannabinoids found in synthetic cannabinoid blends, likely due to the fact that these molecules are easier to synthesize than classical and non-classical cannabinoids. The JWH molecules were first synthesized by John William Huffman at Clemson University
Clemson University () is a public land-grant research university in Clemson, South Carolina. Founded in 1889, Clemson is the second-largest university in the student population in South Carolina. For the fall 2019 semester, the university enro ...
in the late 1990s.FBI
The Federal Bureau of Investigation (FBI) is the domestic Intelligence agency, intelligence and Security agency, security service of the United States and its principal Federal law enforcement in the United States, federal law enforcement age ...
concluded in a 2012 memo that as a result of the publication of J.W. Huffman's research, people searching for a "marijuana-like-high" would follow his recipes and methods.Eicosanoid
Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, around 20 carbon units in length. Eicosanoids are a s ...
synthetic cannabinoids are analogs of endocannabinoids
Cannabinoids () are several structural classes of compounds found in the cannabis plant primarily and most animal organisms (although insects lack such receptors) or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tet ...
, such as anandamide
Anandamide (ANA), also known as ''N''-arachidonoylethanolamine (AEA), is a fatty acid neurotransmitter. Anandamide was the first endocannabinoid to be discovered: it participates in the body's endocannabinoid system by binding to cannabinoid rec ...
. Endocannabinoids
Cannabinoids () are several structural classes of compounds found in the cannabis plant primarily and most animal organisms (although insects lack such receptors) or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tet ...
are cannabinoids naturally occurring in the body. One of the best known synthetic analogs of anandamide is methanandamide.APINACA
APINACA (AKB48, ''N''-(1-adamantyl)-1-pentyl-1''H''-indazole-3-carboxamide) is a drug that acts as a reasonably potent agonist for the cannabinoid receptors, with a Ki of 304.5nM and an EC50 of 585nM at CB1. It had never previously been report ...
(AKB-48), an adamantyl indazole carboxamide, and AB-PINACA
AB-PINACA is a compound that was first identified as a component of synthetic cannabis products in Japan in 2012.
It was originally developed by Pfizer in 2009 as an analgesic medication.
AB-PINACA acts as a potent agonist for the CB1 recepto ...
, an aminocarbonyl indazole carboxamide, is an example of a new group of synthetic cannabinoids.AM-2201
AM-2201 (1-(5-fluoropentyl)-3-(1-naphthoyl)indole) is a recreational designer drug that acts as a potent but nonselective full agonist for the cannabinoid receptor. It is part of the AM series of cannabinoids discovered by Alexandros Makriyanni ...
to THJ-2201
THJ-2201 is an indazole-based synthetic cannabinoid that presumably acts as a potent agonist of the CB1 receptor and has been sold online as a designer drug.
It is a structural analog of AM-2201 in which the central indole ring has been rep ...
) or terminal fluorine replacement;PB-22
PB-22 (QUPIC, SGT-21 or 1-pentyl-1''H''-indole-3-carboxylic acid 8-quinolinyl ester) is a designer drug offered by online vendors as a cannabimimetic agent, and detected being sold in synthetic cannabis products in Japan in 2013. PB-22 represent ...
and 5F-PB-22 were the first synthetic cannabinoids to include a quinoline
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only sli ...
substructure and an ester linkage. These compounds are thought to have been synthesized with the intention of making a synthetic cannabinoid prodrug
A prodrug is a medication or compound that, after intake, is metabolized (i.e., converted within the body) into a pharmacologically active drug. Instead of administering a drug directly, a corresponding prodrug can be used to improve how the drug ...
, which might improve absorption
Absorption may refer to:
Chemistry and biology
* Absorption (biology), digestion
**Absorption (small intestine)
*Absorption (chemistry), diffusion of particles of gas or liquid into liquid or solid materials
*Absorption (skin), a route by which ...
and confound detection. Ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
bonds are easily biodegradable
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegradati ...
through spontaneous or endogenous
Endogenous substances and processes are those that originate from within a living system such as an organism, tissue, or cell.
In contrast, exogenous substances and processes are those that originate from outside of an organism.
For example, es ...
, nonspecific esterase hydrolysis, which has been commonly used in medicinal chemistry to make ester prodrug
A prodrug is a medication or compound that, after intake, is metabolized (i.e., converted within the body) into a pharmacologically active drug. Instead of administering a drug directly, a corresponding prodrug can be used to improve how the drug ...
s. The reason for the change to the quinolone substructure is unknown, but it may have been found to be a suitable replacement for the naphthoyl moiety that is currently regulated by US scheduling laws.non-polar
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end.
Polar molecules must contain one or more pola ...
, small molecules (usually 20-26 carbon atoms) that are fairly volatile, making them "smokable," like THC.psychotropic
A psychoactive drug, psychopharmaceutical, psychoactive agent or psychotropic drug is a chemical substance, that changes functions of the nervous system, and results in alterations in perception, mood, consciousness, cognition or behavior.
Th ...
activity from binding CB1 receptors.agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the ago ...
s of both cannabinoid receptor
Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid recepto ...
s, CB1 and CB2, like THC; however, they often have greater binding affinity
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from ''ligare'', which means 'to bind'. In protein-ligand binding, the ligand is usually a mol ...
and therefore greater potency
Potency may refer to:
* Potency (pharmacology), a measure of the activity of a drug in a biological system
* Virility
* Cell potency, a measure of the differentiation potential of stem cells
* In homeopathic dilutions, potency is a measure of how ...
than THC, as seen in table two. Due to the greater potency
Potency may refer to:
* Potency (pharmacology), a measure of the activity of a drug in a biological system
* Virility
* Cell potency, a measure of the differentiation potential of stem cells
* In homeopathic dilutions, potency is a measure of how ...
, the standard doses of many synthetic cannabinoids may be less than 1 mg.
Stereospecificity
Most classical, non-classical, and hybrid synthetic cannabinoids have stereospecificity
In chemistry, stereospecificity is the property of a reaction mechanism that leads to different stereoisomeric reaction products from different stereoisomeric reactants, or which operates on only one (or a subset) of the stereoisomers."Overlap Co ...
(one stereoisomer is usually much more potent than the ). For example, HU-210
HU-210 is a synthetic cannabinoid that was first synthesized in 1988 from (1R,5S)-myrtenol by a group led by Raphael Mechoulam at the Hebrew University. HU-210 is 100 to 800 times more potent than natural THC from cannabis and has an extended d ...
is the (–) enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...
of 11-OH-Δ8-THC-DMH and a full agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the ago ...
of the CB1 receptor; the (+) enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...
of 11-OH-D8-THC-DMH, known as HU-211, is a NMDA receptor antagonist
NMDA receptor antagonists are a class of drugs that work to antagonize, or inhibit the action of, the ''N''-Methyl-D-aspartate receptor (NMDAR). They are commonly used as anesthetics for animals and humans; the state of anesthesia they induce ...
and is largely inactive as a cannabinoid
Cannabinoids () are several structural classes of compounds found in the cannabis plant primarily and most animal organisms (although insects lack such receptors) or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tet ...
. On the other hand, aminoalkylindole Aminoalkylindoles (AAIs) are a family of cannabinergic compound that act as a cannabinoid receptor agonist. They were invented by pharmaceutical company Sterling-Winthrop in the early 1990s as potential nonsteroidal anti-inflammatory agents.
Leg ...
s, eicosanoid
Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, around 20 carbon units in length. Eicosanoids are a s ...
s, and the other new synthetic cannabinoid groups typically do not have an asymmetric center, so they are usually not stereospecific.
Fluorination of terminal carbon
Recently there has been an increase in the emergence of terminally fluorinated synthetic cannabinoids, such as 5F-PB-22 (fluorinated version of PB-22
PB-22 (QUPIC, SGT-21 or 1-pentyl-1''H''-indole-3-carboxylic acid 8-quinolinyl ester) is a designer drug offered by online vendors as a cannabimimetic agent, and detected being sold in synthetic cannabis products in Japan in 2013. PB-22 represent ...
) and XLR-11
XLR-11 (5"-fluoro-UR-144 or 5F-UR-144) is a drug that acts as a potent agonist for the cannabinoid receptors CB1 and CB2 with EC50 values of 98 nM and 83 nM, respectively. It is a 3-(tetramethylcyclopropylmethanoyl)indole derivative related t ...
(fluorinated version of UR-144
UR-144 (TMCP-018, KM-X1, MN-001, YX-17) is a drug invented by Abbott Laboratories, that acts as a selective full agonist of the peripheral cannabinoid receptor CB2, but with much lower affinity for the psychoactive CB1 receptor.
Pharmacology
...
). South Korea's National Forensic Service reported that 90% of all seized synthetic cannabinoids in 2013 were fluorinated, compared to no fluorinated synthetic cannabinoids reported in 2010. 5F-derivations (terminal fluorination) of the synthetic cannabinoids have been found to be about 2-5 times more potent at CB1 receptors than their un-fluorinated counterparts,
Detection in bodily fluids
Synthetic cannabinoids are typically not identified by the standard marijuana drug tests including the immunoassay test ( EMIT), GC-MS screening, and multi-target screening by LC-GC/MS because those tests only detect the presence of THC
Tetrahydrocannabinol (THC) is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term ''THC' ...
and its metabolites. Although most synthetic cannabinoids are analogs of THC
Tetrahydrocannabinol (THC) is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term ''THC' ...
, they are structurally different enough that, for example, the specific antibodies
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the ...
in the EMIT for marijuana
Cannabis, also known as marijuana among other names, is a psychoactive drug from the cannabis plant. Native to Central or South Asia, the cannabis plant has been used as a drug for both recreational and entheogenic purposes and in various tra ...
do not bind to them. Also, due to their high potency
Potency may refer to:
* Potency (pharmacology), a measure of the activity of a drug in a biological system
* Virility
* Cell potency, a measure of the differentiation potential of stem cells
* In homeopathic dilutions, potency is a measure of how ...
, a very small dose of synthetic cannabinoids is used; moreover, synthetic cannabinoids are highly metabolized by the body, so the window to detect the parent drug (the synthetic cannabinoid itself) in blood and oral fluid is very small.glucuronide conjugation
A glucuronide, also known as glucuronoside, is any substance produced by linking glucuronic acid to another substance via a glycosidic bond. The glucuronides belong to the glycosides.
Glucuronidation, the conversion of chemical compounds to glucu ...
and also by N-dealkylation and aromatic hydroxylation. For example, the main metabolites of JWH-018
JWH-018 (1-pentyl-3-(1-naphthoyl)indole, NA-PIMO or AM-678) is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 cannabinoid receptors, with some selectivity for CB2. It produces effects in ...
, of which there are over 20, include carboxylated, monohydroxylated, dihydroxylated, and trihydroxylated metabolites, but they are mostly excreted in urine as glucuronide conjugates.liquid chromatography-mass spectrometry
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, an ...
is most often used for confirmation and quantitation. There are commercially available EMIT kits for the screening of the synthetic cannabinoids JWH-018
JWH-018 (1-pentyl-3-(1-naphthoyl)indole, NA-PIMO or AM-678) is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 cannabinoid receptors, with some selectivity for CB2. It produces effects in ...
, JWH-073
JWH-073, a synthetic cannabinoid, is an analgesic chemical from the naphthoylindole family that acts as a partial agonist at both the CB1 and CB2 cannabinoid receptors. It is somewhat selective for the CB1 subtype, with affinity at this subtype a ...
, JWH-398
JWH-398 is an analgesic chemical from the naphthoylindole family, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors. It has mild selectivity for CB1 with a Ki of 2.3 nM and 2.8 nM at CB2. This synthetic chemical comp ...
, JWH-200
JWH-200 (WIN 55,225) is an analgesic chemical from the aminoalkylindole family that acts as a cannabinoid receptor agonist. Its binding affinity, ''K''i at the CB1 receptor is 42 nM, around the same as that of THC, but its analgesic potenc ...
, JWH-019
JWH-019 is an analgesic chemical from the naphthoylindole family that acts as a cannabinoid agonist at both the CB1 and CB2 receptors. It is the ''N''-hexyl homolog of the more common synthetic cannabinoid compound JWH-018. Unlike the butyl ho ...
, JWH-122
JWH-122 is a synthetic cannabimimetic that was discovered by John W. Huffman. It is a methylated analogue of JWH-018. It has a Ki of 0.69 nM at CB1 and 1.2 nM at CB2.
In January 2015, over 40 people were reportedly sickened after eating a holid ...
, JWH-081
JWH-081 is an analgesic chemical from the naphthoylindole family, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors. With a Ki of 1.2nM it is fairly selective for the CB1 subtype, its affinity at this subtype is measured at ap ...
, JWH-250
JWH-250 or (1-pentyl-3-(2-methoxyphenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family that acts as a cannabinoid agonist at both the CB1 and CB2 receptors, with a ''K''i of 11 nM at CB1 and 33 nM at CB2. U ...
, JWH-203
JWH-203 (1-pentyl-3-(2-chlorophenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family that acts as a cannabinoid agonist with approximately equal affinity at both the CB1 and CB2 receptors, having a Ki of 8.0 nM at ...
, CP-47,497
CP 47,497 or (C7)-CP 47,497 is a cannabinoid receptor agonist drug, developed by Pfizer in the 1980s. It has analgesic effects and is used in scientific research. It is a potent CB1 agonist with a ''K''d of 2.1 nM.
Homologue
On the 19th of ...
, CP-47,497-C8, HU-210
HU-210 is a synthetic cannabinoid that was first synthesized in 1988 from (1R,5S)-myrtenol by a group led by Raphael Mechoulam at the Hebrew University. HU-210 is 100 to 800 times more potent than natural THC from cannabis and has an extended d ...
, HU-211, AM-2201
AM-2201 (1-(5-fluoropentyl)-3-(1-naphthoyl)indole) is a recreational designer drug that acts as a potent but nonselective full agonist for the cannabinoid receptor. It is part of the AM series of cannabinoids discovered by Alexandros Makriyanni ...
, AM-694
AM-694 (1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole) is a designer drug that acts as a potent and selective agonist for the cannabinoid receptor CB1. It is used in scientific research for mapping the distribution of CB1 receptors.
Pharmacology
A ...
, RCS-4
RCS-4, or 1-pentyl-3-(4-methoxybenzoyl)indole, is a synthetic cannabinoid drug sold under the names SR-19, BTM-4, or Eric-4 (later shortened to E-4), but originally, OBT-199.
Pharmacology
RCS-4 is a potent cannabinoid receptor agonist, with EC ...
, and RCS-8 through companies like NMS Labs, Cayman Chemical, and Immunoanalysis Corporation.
Notable incidents
On October 20, 2011, the Louisiana State University football program announced that it had suspended three players, including star cornerback Tyrann Mathieu
Tyrann Devine Mathieu (; born May 13, 1992) is an American football safety for the New Orleans Saints of the National Football League (NFL). He played college football at LSU. In college he developed a reputation for causing turnovers, setting a ...
, who tested positive for synthetic cannabinoids.
In the autumn of 2014, more than two thousand Spice consumers in Russia sought medical attention, one-thousand were admitted to hospitals, and 40 people died.overdosed
A drug overdose (overdose or OD) is the ingestion or application of a drug or other substance in quantities much greater than are recommended. , in Brooklyn
Brooklyn () is a borough of New York City, coextensive with Kings County, in the U.S. state of New York. Kings County is the most populous county in the State of New York, and the second-most densely populated county in the United States, be ...
. 18 people were transported to local hospitals. The herbal "incense" product was determined to be a synthetic cannabinoid called AMB-FUBINACA
AMB-FUBINACA (also known as FUB-AMB and MMB-FUBINACA) is an indazole-based synthetic cannabinoid that is a potent agonist for the cannabinoid receptors, with ''K''i values of 10.04 nM at CB1 and 0.786 nM at CB2 and EC50 values of 0. ...
.brodifacoum
Brodifacoum is a highly lethal 4-hydroxycoumarin vitamin K antagonist anticoagulant poison. In recent years, it has become one of the world's most widely used pesticides. It is typically used as a rodenticide, but is also used to control larger ...
, a rat poison that causes bleeding. Illinois was hit the hardest and on April 5, 2018, the CDC
The Centers for Disease Control and Prevention (CDC) is the national public health agency of the United States. It is a United States federal agency, under the Department of Health and Human Services, and is headquartered in Atlanta, Georgi ...
issued a Clinical Action alert to health care providers across the United States advising of 89 confirmed cases of "serious unexplained bleeding" in Illinois. The cases are still being studied; however, 63 of the patients reported synthetic cannabinoid use, and laboratory analysis confirmed brodifacoum
Brodifacoum is a highly lethal 4-hydroxycoumarin vitamin K antagonist anticoagulant poison. In recent years, it has become one of the world's most widely used pesticides. It is typically used as a rodenticide, but is also used to control larger ...
was present in at least 18 patients. As of April 24, 2018, 153 cases, including four deaths, linked to this outbreak have been reported to the Illinois Department of Public Health
The Illinois Department of Public Health (IDPH) is the code department of the Illinois state government that prevents and controls disease and injury, regulates medical practitioners, and promotes sanitation.
IDPH offices
The Illinois Department ...
(IDPH) since March 7, 2018. On September 18, 2018, the Wisconsin Department of Health Services
The Wisconsin Department of Health Services (WisDHS) is a governmental agency of the U.S. state of Wisconsin responsible for maintaining public health. It administers a wide range of services in the state and at state institutions, regulates hosp ...
confirmed 16 more cases, bringing the total number of people affected by the outbreak in Wisconsin to 80 people since March 2018, including one death in July 2018.
In August 2018, there were almost one hundred overdose cases reported over two days in New Haven, Connecticut from a bad batch of K2. The synthetic cannabinoid was believed to have been mixed with fentanyl
Fentanyl, also spelled fentanil, is a very potent synthetic opioid used as a pain medication. Together with other drugs, fentanyl is used for anesthesia. It is also used illicitly as a recreational drug, sometimes mixed with heroin, cocaine ...
, although no fentanyl
Fentanyl, also spelled fentanil, is a very potent synthetic opioid used as a pain medication. Together with other drugs, fentanyl is used for anesthesia. It is also used illicitly as a recreational drug, sometimes mixed with heroin, cocaine ...
was identified in samples of the drug tested by the DEA
The Drug Enforcement Administration (DEA; ) is a United States federal law enforcement agency under the U.S. Department of Justice tasked with combating drug trafficking and distribution within the U.S. It is the lead agency for domestic en ...
.
In September 2018, at least 10 people overdosed on a synthetic cannabinoid, either AMB-FUBINACA
AMB-FUBINACA (also known as FUB-AMB and MMB-FUBINACA) is an indazole-based synthetic cannabinoid that is a potent agonist for the cannabinoid receptors, with ''K''i values of 10.04 nM at CB1 and 0.786 nM at CB2 and EC50 values of 0. ...
or AB, in Christchurch
Christchurch ( ; mi, Ōtautahi) is the largest city in the South Island of New Zealand and the seat of the Canterbury Region. Christchurch lies on the South Island's east coast, just north of Banks Peninsula on Pegasus Bay. The Avon River / ...
, New Zealand
New Zealand ( mi, Aotearoa ) is an island country in the southwestern Pacific Ocean. It consists of two main landmasses—the North Island () and the South Island ()—and over 700 smaller islands. It is the sixth-largest island count ...
over two days. Some of the people are in critical condition in the Intensive Care Unit
220px, Intensive care unit
An intensive care unit (ICU), also known as an intensive therapy unit or intensive treatment unit (ITU) or critical care unit (CCU), is a special department of a hospital or health care facility that provides intensiv ...
.
From September 21–22, 2018, almost 50 people overdosed and two people died in the Kensington
Kensington is a district in the Royal Borough of Kensington and Chelsea in the West End of London, West of Central London.
The district's commercial heart is Kensington High Street, running on an east–west axis. The north-east is taken up b ...
area of Philadelphia
Philadelphia, often called Philly, is the largest city in the Commonwealth of Pennsylvania, the sixth-largest city in the U.S., the second-largest city in both the Northeast megalopolis and Mid-Atlantic regions after New York City. Sinc ...
. Officials believed the cause to be a combination of heroin
Heroin, also known as diacetylmorphine and diamorphine among other names, is a potent opioid mainly used as a recreational drug for its euphoric effects. Medical grade diamorphine is used as a pure hydrochloride salt. Various white and brow ...
or fentanyl
Fentanyl, also spelled fentanil, is a very potent synthetic opioid used as a pain medication. Together with other drugs, fentanyl is used for anesthesia. It is also used illicitly as a recreational drug, sometimes mixed with heroin, cocaine ...
and a synthetic cannabinoid. This same area in Philadelphia
Philadelphia, often called Philly, is the largest city in the Commonwealth of Pennsylvania, the sixth-largest city in the U.S., the second-largest city in both the Northeast megalopolis and Mid-Atlantic regions after New York City. Sinc ...
had 155 people overdose and 10 people die from a combination of heroin
Heroin, also known as diacetylmorphine and diamorphine among other names, is a potent opioid mainly used as a recreational drug for its euphoric effects. Medical grade diamorphine is used as a pure hydrochloride salt. Various white and brow ...
, fentanyl
Fentanyl, also spelled fentanil, is a very potent synthetic opioid used as a pain medication. Together with other drugs, fentanyl is used for anesthesia. It is also used illicitly as a recreational drug, sometimes mixed with heroin, cocaine ...
, and a synthetic cannabinoid called 5F-ADB
5F-ADB (also known as 5F-MDMB-PINACA) is an indazole-based synthetic cannabinoid from the indazole-3-carboxamide family, which has been used as an active ingredient in synthetic cannabis products and has been sold online as a designer drug. 5F-A ...
over one weekend in July 2018. The Department of Public Health released that they believe "5F-ADB
5F-ADB (also known as 5F-MDMB-PINACA) is an indazole-based synthetic cannabinoid from the indazole-3-carboxamide family, which has been used as an active ingredient in synthetic cannabis products and has been sold online as a designer drug. 5F-A ...
was the primary cause of the cluster of patients with these adverse drug reactions."
On December 10, 2021, the Hillsborough County, Florida department of health reported cases of "rat poison" contaminated synthetic blends linked to symptoms associated with coagulopathy, a condition where the blood's ability to clot is impaired. 2 deaths and over 41 hospitalizations have been directly linked to this specific outbreak as of December 16, 2021.
Research
Vaping-associated pulmonary injury
Synthetic cannabinoids have been speculated to be involved in vaping-associated pulmonary injury
Vaping-associated pulmonary injury (VAPI), also known as vaping-associated lung injury (VALI) or e-cigarette, or vaping, product use associated lung injury (E/VALI), is an umbrella term, used to describe lung diseases associated with the use of v ...
(VAPI).
Legal restrictions and regional availability
Europe
Austria
The Austrian Ministry of Health announced on December 18, 2008, that Spice would be controlled under Paragraph 78 of their drug-law on the grounds that it contains an active substance that affects the functions of the body, and the legality of JWH-018 is under review.
Germany
JWH-018, CP 47,497 and the C6, C8, and C9 homologues of CP 47,497 have been illegal in Germany since January 22, 2009.
France
JWH-018, CP 47,497 (and its homologues), and HU-210 were all made illegal in France on February 24, 2009.
Ireland
From June 2010, JWH-018, along with a variety of other designer drugs, has been illegal.
Latvia
 JWH-018, JWH-073, CP 47,497 (and its homologues), and HU-210 as well as ''
JWH-018, JWH-073, CP 47,497 (and its homologues), and HU-210 as well as ''leonotis leonurus
''Leonotis leonurus'', also known as lion's tail and wild dagga, is a plant species in the mint family, Lamiaceae. The plant is a broadleaf evergreen large shrub native to South Africa and southern Africa, where it is very common.[JWH-018
JWH-018 (1-pentyl-3-(1-naphthoyl)indole, NA-PIMO or AM-678) is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 cannabinoid receptors, with some selectivity for CB2. It produces effects in ...]
and many of the herbs mentioned on the ingredient lists of Spice and similar preparations were made illegal in May 2009. The bill was passed by Polish Sejm
The Sejm (English: , Polish: ), officially known as the Sejm of the Republic of Poland (Polish: ''Sejm Rzeczypospolitej Polskiej''), is the lower house of the bicameral parliament of Poland.
The Sejm has been the highest governing body of the ...
and Polish Senat
The Senate ( pl, Senat) is the upper house of the Polish parliament, the lower house being the Sejm. The history of the Polish Senate stretches back over 500 years; it was one of the first constituent bodies of a bicameral parliament in Europe ...
and was signed by the President.
Romania
Spice was made illegal in Romania on February 15, 2010. As on 12 September 2018 Spice was made legal for personal use. A new law is being discussed to make spice illegal for personal use again.
Russia
On April 9, 2009, the Chief Medical Officer of the Russian Federation issued a resolution on reinforcing control over the sales of smoking-blends. These blends, marketed under the trade names AM-HI-CO, Dream, Spice (Gold, Diamond), Zoom, Ex-ses, Yucatán Fire and others, have been declared to contain ''Salvia divinorum
''Salvia divinorum'' (Latin: "sage of the diviners"; also called ska maría pastora, seer's sage, yerba de la pastora, magic mint or simply salvia) is a plant species with transient psychoactive properties when its leaves are consumed by che ...
'', Hawaiian wood rose, and blue lotus, and are prohibited to be sold. These substances have been found to have "psychotropic, narcotic effects, contain poisonous components and represent potential threat for humans". The resolution does not mention JWH-018 or other synthetic cannabinoids. On January 14, 2010, the Russian government issued a statement including 23 synthetic cannabinoids found in smoking blends Hawaiian Rose and Blue Lotus on the list of prohibited narcotic and psychotropic substances.
About 780 new psychoactive substances were added to the list from 2011 to 2014. The drug-makers avoided all the bans by making slight changes to the drugs. In the autumn of 2014, more than two-thousand Spice consumers in Russia sought medical attention, one-thousand were admitted to hospitals, and 40 people diedVladimir Putin
Vladimir Vladimirovich Putin; (born 7 October 1952) is a Russian politician and former intelligence officer who holds the office of president of Russia. Putin has served continuously as president or prime minister since 1999: as prime min ...
brought in a bill that increased the penalty for selling or consuming smoking blends from a fine to up to eight years in prison.
Slovakia
Spice is legal in Slovakia. The National Anti-Drug Unit is considering adding it to the list of controlled substances. The latest anti-drug law version (468/2009) valid since January 2010 does not mention active compounds of Spice.
Spain
Spice is unregulated in Spain. For this reason, Spice is available in grow shop stores or cannabis related stores, and it can be bought and shipped online without any legal impediment from those kind of stores.
Sweden
CP 47,497-C6, CP 47,497-C7, CP 47,497-C8, CP 47,497-C9, JWH-018, JWH-073
JWH-073, a synthetic cannabinoid, is an analgesic chemical from the naphthoylindole family that acts as a partial agonist at both the CB1 and CB2 cannabinoid receptors. It is somewhat selective for the CB1 subtype, with affinity at this subtype a ...
, and HU-210
HU-210 is a synthetic cannabinoid that was first synthesized in 1988 from (1R,5S)-myrtenol by a group led by Raphael Mechoulam at the Hebrew University. HU-210 is 100 to 800 times more potent than natural THC from cannabis and has an extended d ...
were all made illegal in Sweden on September 15, 2009. The bill was accepted on July 30, 2009, and was put in effect on September 15, 2009.
Switzerland
Spice has been banned in Switzerland.
Turkey
Spice, which is colloquially called ''bonzai'' in Turkey, was added to the list of drugs and psychotropic substances on July 1, 2011, by the law numbered as 2011–1310 B.K.K. (February 13, 2011 and the Official Gazette No. 27845).
United Kingdom
The UK controls synthetic cannabinoids by analog under the Misuse of Drugs Act, 1971 as Class B drugs. Until 2016, synthetic cannabinoids were legally sold in head shop
A head shop is a retail outlet specializing in paraphernalia used for consumption of cannabis and tobacco and items related to cannabis culture and related countercultures. They emerged from the hippie counterculture in the late 1960s, and ...
s, although the exact compounds available changed over time based on the legislation. The UK saw three generations of synthetic cannabinoids within five years where the second and third generations emerged in response to amendments to the Misuse of Drugs Act, 1971, Order 2009 and Order 2013, which classified many first and second generation synthetic cannabinoids as Class B drugs. There were two additional amendments in 2016 and 2019, which included in the analog controls many of the most popular synthetic cannabinoids circulating at the time. In May 2016, the Psychoactive Substances Act was enacted, which made illegal the production, distribution, sale, supply, and possession in correctional institutions
A prison, also known as a jail, gaol (dated, standard English, Australian, and historically in Canada), penitentiary (American English and Canadian English), detention center (or detention centre outside the US), correction center, correcti ...
of any substance for human consumption with psychoactive effects. This stopped the open sale of synthetic cannabinoids in head shop
A head shop is a retail outlet specializing in paraphernalia used for consumption of cannabis and tobacco and items related to cannabis culture and related countercultures. They emerged from the hippie counterculture in the late 1960s, and ...
s, although they are still found in use.
North America
Canada
Spice is not specifically prohibited in Canada, but synthetic cannabis mimics are listed as a schedule II drug. Schedule II to the Controlled Drugs and Substances Act makes reference to specific synthetic compounds JWH-XXX and AM-XXXX, although is not limiting to those identified. Health Canada
Health Canada (HC; french: Santé Canada, SC)Health Canada is the applied title under the Federal Identity Program; the legal title is Department of Health (). is the Structure of the Canadian federal government#Departments, with subsidiary unit ...
is debating the subject. Schedule II has consisted entirely of synthetic cannabinoids since October 2018; these remain illegal following the removal from the schedule of cannabis and its constituents derived from nature.
United States
The case of David Mitchell Rozga, an American teenager from Indianola, Iowa
Indianola is a city in Warren County, Iowa, United States, located south of downtown Des Moines, Iowa. The population was 15,833 at the time of the 2020 census. It is the county seat of Warren County. Indianola is home to the National Balloon ...
, brought international attention to K2. Rozga shot himself in the head with a family-owned hunting rifle
A rifle is a long-barreled firearm designed for accurate shooting, with a barrel that has a helical pattern of grooves (rifling) cut into the bore wall. In keeping with their focus on accuracy, rifles are typically designed to be held with bo ...
in an apparent suicide
Suicide is the act of intentionally causing one's own death. Mental disorders (including depression, bipolar disorder, schizophrenia, personality disorders, anxiety disorders), physical disorders (such as chronic fatigue syndrome), and s ...
on June 6, 2010. After news of Rozga's death, it was reported by friends that they had smoked K2 with Rozga approximately one hour before his death. The nature of his death and reports from numerous family members, led investigators to suspect that Rozga was under the influence of a mind-altering substance when he died. The death of Rozga influenced political lobbying against K2, and other legal synthetic drugs such as bath salts
Bath salts are water-soluble, pulverized minerals that are added to water to be used for bathing. They are said to improve cleaning, enhance the enjoyment of bathing, and serve as a vehicle for cosmetic agents. Bath salts have been developed wh ...
. Following the incident, the "David Mitchell Rozga Act" to ban the use and distribution of K2 was introduced by Iowa Senator Chuck Grassley
Charles Ernest Grassley (born September 17, 1933) is an American politician serving as the president pro tempore emeritus of the United States Senate, and the Seniority in the United States Senate, senior United States Senate, United States sen ...
. It was passed by the United States Congress
The United States Congress is the legislature of the federal government of the United States. It is bicameral, composed of a lower body, the House of Representatives, and an upper body, the Senate. It meets in the U.S. Capitol in Washing ...
in June 2011. On July 10, 2012, President Barack Obama
Barack Hussein Obama II ( ; born August 4, 1961) is an American politician who served as the 44th president of the United States from 2009 to 2017. A member of the Democratic Party, Obama was the first African-American president of the U ...
signed the Synthetic Drug Abuse Prevention Act of 2012
The Food and Drug Administration Safety and Innovation Act of 2012 (FDASIA) is a piece of American regulatory legislation signed into law on July 9, 2012. It gives the United States Food and Drug Administration (FDA) the authority to collect user ...
into law. It banned synthetic compounds commonly found in synthetic marijuana, placing them under Schedule I of the Controlled Substances Act
The Controlled Substances Act (CSA) is the statute establishing federal government of the United States, federal drug policy of the United States, U.S. drug policy under which the manufacture, importation, possession, use, and distribution of ...
.Prior to that, some synthetic cannabis compounds (
HU-210
HU-210 is a synthetic cannabinoid that was first synthesized in 1988 from (1R,5S)-myrtenol by a group led by Raphael Mechoulam at the Hebrew University. HU-210 is 100 to 800 times more potent than natural THC from cannabis and has an extended d ...
) were scheduled in the US under federal law, while others (JWH-073
JWH-073, a synthetic cannabinoid, is an analgesic chemical from the naphthoylindole family that acts as a partial agonist at both the CB1 and CB2 cannabinoid receptors. It is somewhat selective for the CB1 subtype, with affinity at this subtype a ...
) were temporarily scheduled until final determination of their status could be made. The Drug Enforcement Administration
The Drug Enforcement Administration (DEA; ) is a Federal law enforcement in the United States, United States federal law enforcement agency under the U.S. Department of Justice tasked with combating drug trafficking and distribution within th ...
(DEA) considered K2 to be a "drug of concern", citing "...a surge in emergency-room visits and calls to poison-control centers. Adverse health effects associated with its use include seizures, hallucinations, paranoid behavior, agitation, anxiety, nausea, vomiting, racing heartbeat, and elevated blood pressure."
Several states independently passed acts making it illegal under state law, including Kansas
Kansas () is a state in the Midwestern United States. Its capital is Topeka, and its largest city is Wichita. Kansas is a landlocked state bordered by Nebraska to the north; Missouri to the east; Oklahoma to the south; and Colorado to the ...
in March 2010,[The Associated Press. How major issues fared in Kansas Legislature. CNBC. May 23, 2010. Accessed: May 23, 2010](_blank)
/ref> Georgia
Georgia most commonly refers to:
* Georgia (country), a country in the Caucasus region of Eurasia
* Georgia (U.S. state), a state in the Southeast United States
Georgia may also refer to:
Places
Historical states and entities
* Related to the ...
and Alabama
(We dare defend our rights)
, anthem = "Alabama (state song), Alabama"
, image_map = Alabama in United States.svg
, seat = Montgomery, Alabama, Montgomery
, LargestCity = Huntsville, Alabama, Huntsville
, LargestCounty = Baldwin County, Al ...
in May 2010,Tennessee
Tennessee ( , ), officially the State of Tennessee, is a landlocked state in the Southeastern region of the United States. Tennessee is the 36th-largest by area and the 15th-most populous of the 50 states. It is bordered by Kentucky to th ...
and Missouri
Missouri is a U.S. state, state in the Midwestern United States, Midwestern region of the United States. Ranking List of U.S. states and territories by area, 21st in land area, it is bordered by eight states (tied for the most with Tennessee ...
in July 2010,[Gay, Malcolm. (2010-07-10]
Synthetic Marijuana Spurs State Bans
''New York Times''. Retrieved on August 7, 2011. Louisiana
Louisiana , group=pronunciation (French: ''La Louisiane'') is a state in the Deep South and South Central regions of the United States. It is the 20th-smallest by area and the 25th most populous of the 50 U.S. states. Louisiana is borde ...
in August 2010, Mississippi
Mississippi () is a state in the Southeastern region of the United States, bordered to the north by Tennessee; to the east by Alabama; to the south by the Gulf of Mexico; to the southwest by Louisiana; and to the northwest by Arkansas. Miss ...
in September 2010, and Iowa
Iowa () is a state in the Midwestern region of the United States, bordered by the Mississippi River to the east and the Missouri River and Big Sioux River to the west. It is bordered by six states: Wisconsin to the northeast, Illinois to the ...
. An emergency order was passed in Arkansas
Arkansas ( ) is a landlocked state in the South Central United States. It is bordered by Missouri to the north, Tennessee and Mississippi to the east, Louisiana to the south, and Texas and Oklahoma to the west. Its name is from the Osage ...
in July 2010 banning the sale of synthetic cannabis mimics. In October 2010, the Oregon Board of Pharmacy listed synthetic cannabinoid chemicals on its Schedule 1 of controlled substance, which means that the sale and possession of these substances is illegal under the Oregon Uniform Controlled Substances Act.National Conference of State Legislatures
The National Conference of State Legislatures (NCSL), established in 1975, is a "nonpartisan public officials’ association composed of sitting state legislators" from the states, territories and commonwealths of the United States.
Background ...
, several other states also considered legislation, including New Jersey
New Jersey is a state in the Mid-Atlantic and Northeastern regions of the United States. It is bordered on the north and east by the state of New York; on the east, southeast, and south by the Atlantic Ocean; on the west by the Delaware ...
, New York, Florida, and Ohio
Ohio () is a state in the Midwestern region of the United States. Of the fifty U.S. states, it is the 34th-largest by area, and with a population of nearly 11.8 million, is the seventh-most populous and tenth-most densely populated. The sta ...
.South Dakota
South Dakota (; Sioux language, Sioux: , ) is a U.S. state in the West North Central states, North Central region of the United States. It is also part of the Great Plains. South Dakota is named after the Lakota people, Lakota and Dakota peo ...
Legislature
A legislature is an assembly with the authority to make law
Law is a set of rules that are created and are enforceable by social or governmental institutions to regulate behavior,Robertson, ''Crimes against humanity'', 90. with its p ...
passed a ban on these products which was signed into law by Gov. Dennis Daugaard
Dennis Martin Daugaard (born June 11, 1953) is an American attorney and politician who served as the 32nd governor of South Dakota from 2011 to 2019. A member of the Republican Party, he was the first chief executive of a U.S. state to be the c ...
on February 23, 2012 (and which took immediate effect under an emergency clause of the state constitution). Indiana
Indiana () is a U.S. state in the Midwestern United States. It is the 38th-largest by area and the 17th-most populous of the 50 States. Its capital and largest city is Indianapolis. Indiana was admitted to the United States as the 19th s ...
banned synthetic cannabinoids in a law which became effective in March 2012. North Carolina
North Carolina () is a state in the Southeastern region of the United States. The state is the 28th largest and 9th-most populous of the United States. It is bordered by Virginia to the north, the Atlantic Ocean to the east, Georgia and So ...
banned synthetic cannabis mimics by a unanimous vote of the state senate, due to concerns that its contents and effects are reasonably similar to cannabis, and may cause equal effects in terms of psychological dependency.
Following cases in Japan involving the use of synthetic cannabinoids by navy, army and marine corps personnel, they were officially banned.[Allan, David; Fisher, Cindy. Ruling Clarifies 'Legal' Drug Policy. Stars and Stripes via Military.com. May 23, 2010. Accessed: May 23, 2010](_blank)
A punitive general order issued on January 4, 2010, by the Commander Marine Corps Forces, Pacific prohibits the actual or attempted possession, use, sale, distribution and manufacture of synthetic cannabis mimics as well as any derivative, analogue or variant of it.[Marines Ban Spice Drug. Military.com. May 23, 2010. Accessed: May 23, 2010](_blank)
On June 8, 2010, the US Air Force issued a memorandum that banned the possession and use of Spice, or any other mood-altering substance except alcohol or tobacco, among its service members.
South America
Chile
The Chilean Ministry of Health
Chilean may refer to:
* Something of, from, or related to Chile, a country in South America
* Chilean people
* Chilean Spanish
* Chilean culture
* Chilean cuisine
* Chilean Americans
See also
*List of Chileans
This is a list of Chileans who ar ...
on April 24, 2009, declared the sale of synthetic cannabis mimics to be illegal.
Asia
South Korea
South Korea officially added JWH-018, CP 47,497 and HU-210 to the controlled substance list on July 1, 2009, effectively making these chemicals illegal.
Indonesia
''Tembakau Gorilla'' (Gorilla Tobacco), a catch-all term for synthetic cannabinoids blended in tobacco products, were listed as Class I Narcotics with no therapeutic use in 2017.
Japan
Japan has banned JWH-018, CP 47, 497, and homologues, and HU-210 since October 2009.
United Arab-Emirates
The United Arab Emirates had stated that Spice is an illegal substance and possession or intent to sell is a jailable offense.
Australasia
Australia
On June 17, 2011, the Western Australian government banned all of the synthetic cannabinoids found in already existing products, including brands such as Kronic, Kalma, Voodoo, Kaos, and Mango Kush. Western Australia was the first state in Australia to prohibit the sale of certain synthetic cannabinoids.
New Zealand
Synthetic Cannabinoids are illegal in New Zealand, it is classified as a Class A controlled drug. The New Zealand Parliament passed a law in July 2013 banning the sale of legal highs in dairies
A dairy is a business enterprise established for the harvesting or processing (or both) of animal milk – mostly from cows or buffaloes, but also from goats, sheep, horses, or camels – for human consumption. A dairy is typically located on a ...
and supermarkets, but allowing some "low risk" drugs to continue to be sold through speciality licensed shops. Synthetic cannabinoids, as well as all other legal highs were outlawed at midnight on 7 May 2014, after a law was passed a week prior by the New Zealand government.
An analysis of 41 different synthetic cannabis mimic blends sold commercially in New Zealand
New Zealand ( mi, Aotearoa ) is an island country in the southwestern Pacific Ocean. It consists of two main landmasses—the North Island () and the South Island ()—and over 700 smaller islands. It is the sixth-largest island count ...
, conducted by the Institute of Environmental Science and Research
The Institute of Environmental Science and Research (ESR) is a New Zealand Crown Research Institute (CRI). Its purpose is to deliver scientific and research services to the public health, food safety, security and justice systems, and the enviro ...
and released in July 2011, found 11 different synthetic cannabinoid ingredients used, including JWH-018
JWH-018 (1-pentyl-3-(1-naphthoyl)indole, NA-PIMO or AM-678) is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 cannabinoid receptors, with some selectivity for CB2. It produces effects in ...
, JWH-073
JWH-073, a synthetic cannabinoid, is an analgesic chemical from the naphthoylindole family that acts as a partial agonist at both the CB1 and CB2 cannabinoid receptors. It is somewhat selective for the CB1 subtype, with affinity at this subtype a ...
, AM-694
AM-694 (1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole) is a designer drug that acts as a potent and selective agonist for the cannabinoid receptor CB1. It is used in scientific research for mapping the distribution of CB1 receptors.
Pharmacology
A ...
, AM-2201
AM-2201 (1-(5-fluoropentyl)-3-(1-naphthoyl)indole) is a recreational designer drug that acts as a potent but nonselective full agonist for the cannabinoid receptor. It is part of the AM series of cannabinoids discovered by Alexandros Makriyanni ...
, RCS-4
RCS-4, or 1-pentyl-3-(4-methoxybenzoyl)indole, is a synthetic cannabinoid drug sold under the names SR-19, BTM-4, or Eric-4 (later shortened to E-4), but originally, OBT-199.
Pharmacology
RCS-4 is a potent cannabinoid receptor agonist, with EC ...
, RCS-4 butyl homologue, JWH-210
JWH-210 is an analgesic chemical from the naphthoylindole family, which acts as a potent cannabinoid agonist at both the CB1 and CB2 receptors, with Ki values of 0.46 nM at CB1 and 0.69 nM at CB2. It is one of the most potent 4-substi ...
, JWH-081
JWH-081 is an analgesic chemical from the naphthoylindole family, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors. With a Ki of 1.2nM it is fairly selective for the CB1 subtype, its affinity at this subtype is measured at ap ...
, JWH-250
JWH-250 or (1-pentyl-3-(2-methoxyphenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family that acts as a cannabinoid agonist at both the CB1 and CB2 receptors, with a ''K''i of 11 nM at CB1 and 33 nM at CB2. U ...
(or possibly JWH-302
JWH-302 (1-pentyl-3-(3-methoxyphenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family, which acts as a cannabinoid agonist with moderate affinity at both the CB1 and CB2 receptors. It is a positional isomer of the more ...
, isomer not determined), JWH-203
JWH-203 (1-pentyl-3-(2-chlorophenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family that acts as a cannabinoid agonist with approximately equal affinity at both the CB1 and CB2 receptors, having a Ki of 8.0 nM at ...
, and JWH-122
JWH-122 is a synthetic cannabimimetic that was discovered by John W. Huffman. It is a methylated analogue of JWH-018. It has a Ki of 0.69 nM at CB1 and 1.2 nM at CB2.
In January 2015, over 40 people were reportedly sickened after eating a holid ...
—with between one and five different active ingredients, though JWH-018 was present in 37 of the 41 blends tested. In two brands, the benzodiazepine
Benzodiazepines (BZD, BDZ, BZs), sometimes called "benzos", are a class of depressant drugs whose core chemical structure is the fusion of a benzene ring and a diazepine ring. They are prescribed to treat conditions such as anxiety disorders, ...
anxiolytic
An anxiolytic (; also antipanic or antianxiety agent) is a medication or other intervention that reduces anxiety. This effect is in contrast to anxiogenic agents which increase anxiety. Anxiolytic medications are used for the treatment of anxi ...
drug phenazepam
Phenazepam (also known in Russia as bromdihydrochlorphenylbenzodiazepine) is a benzodiazepine drug, which was developed in the Soviet Union in 1975, and now produced in Russia and some CIS countries.
Phenazepam is used in the treatment of vari ...
was also found, which is classified as a prescription medicine
A prescription drug (also prescription medication or prescription medicine) is a pharmaceutical drug that legally requires a medical prescription to be dispensed. In contrast, over-the-counter drugs can be obtained without a prescription. The rea ...
in New Zealand, and these brands were ordered to be removed from the market by emergency recall.temporary class drug
A temporary class drug is a relatively new status for controlled drugs, which has been adopted in some jurisdictions, notably New Zealand and the United Kingdom, to attempt to bring newly synthesised designer drugs under legal control. The control ...
s. In 2013, another hypnotic medication, zaleplon
Zaleplon, sold under the brand names Sonata among others, is a sedative-hypnotic, used to treat insomnia. It is a nonbenzodiazepine hypnotic from the pyrazolopyrimidine class.
It is manufactured by King Pharmaceuticals and Gedeon Richter Plc. ...
, was found to have been used as an active ingredient in a blend that had been sold in New Zealand during 2011 and 2012.
See also
* List of designer drugs § Synthetic cannabinoids
*Endocannabinoid enhancer
An endocannabinoid enhancer (eCBE) is a type of cannabinoidergic drug that enhances the activity of the endocannabinoid system by increasing extracellular concentrations of endocannabinoids. Examples of different types of eCBEs include fatty acid ...
*Endocannabinoid system
The endocannabinoid system (ECS) is a biological system composed of endocannabinoids, which are endogenous lipid-based retrograde neurotransmitters that bind to cannabinoid receptors (CBRs), and cannabinoid receptor proteins that are expressed ...
*Structural scheduling of synthetic cannabinoids To combat the illicit synthetic cannabinoid industry many jurisdictions have created a system to control these cannabinoids through their general (or Markush) structure as opposed to their specific identity. In this way new analogs are already cont ...
References
External links
ErowidSynthetic cannabinoid profile
at the European Monitoring Centre for Drugs and Drug Addiction
The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) is an agency of the European Union located in Lisbon, Portugal, and established in 1993. In June 2022, the Council of the European Union approved a reform of the organization w ...
Interactive model to help understand the structures of synthetic cannabinoids
{{DEFAULTSORT:Spice (Drug)
*
Designer drugs
Drug culture
 Synthetic cannabinoids are a class of
Synthetic cannabinoids are a class of  * Counterfeit cannabis-liquid (c-liquid) for
* Counterfeit cannabis-liquid (c-liquid) for 
 There are five major categories for synthetic cannabinoids: classical cannabinoids, non-classical cannabinoids, hybrid cannabinoids,
There are five major categories for synthetic cannabinoids: classical cannabinoids, non-classical cannabinoids, hybrid cannabinoids,  JWH-018, JWH-073, CP 47,497 (and its homologues), and HU-210 as well as ''
JWH-018, JWH-073, CP 47,497 (and its homologues), and HU-210 as well as ''