Salen complex on:
[Wikipedia]
[Google]
[Amazon]
A metal salen complex is a coordination compound between a metal cation and a  The metal-free salen compound (H2salen or salenH2) has two
The metal-free salen compound (H2salen or salenH2) has two
 Salen complexes with ''d''8 metal ions, such as Ni(salen), typically have a low-spin
Salen complexes with ''d''8 metal ions, such as Ni(salen), typically have a low-spin

 Unsubstituted salen complexes are poorly soluble in
Unsubstituted salen complexes are poorly soluble in 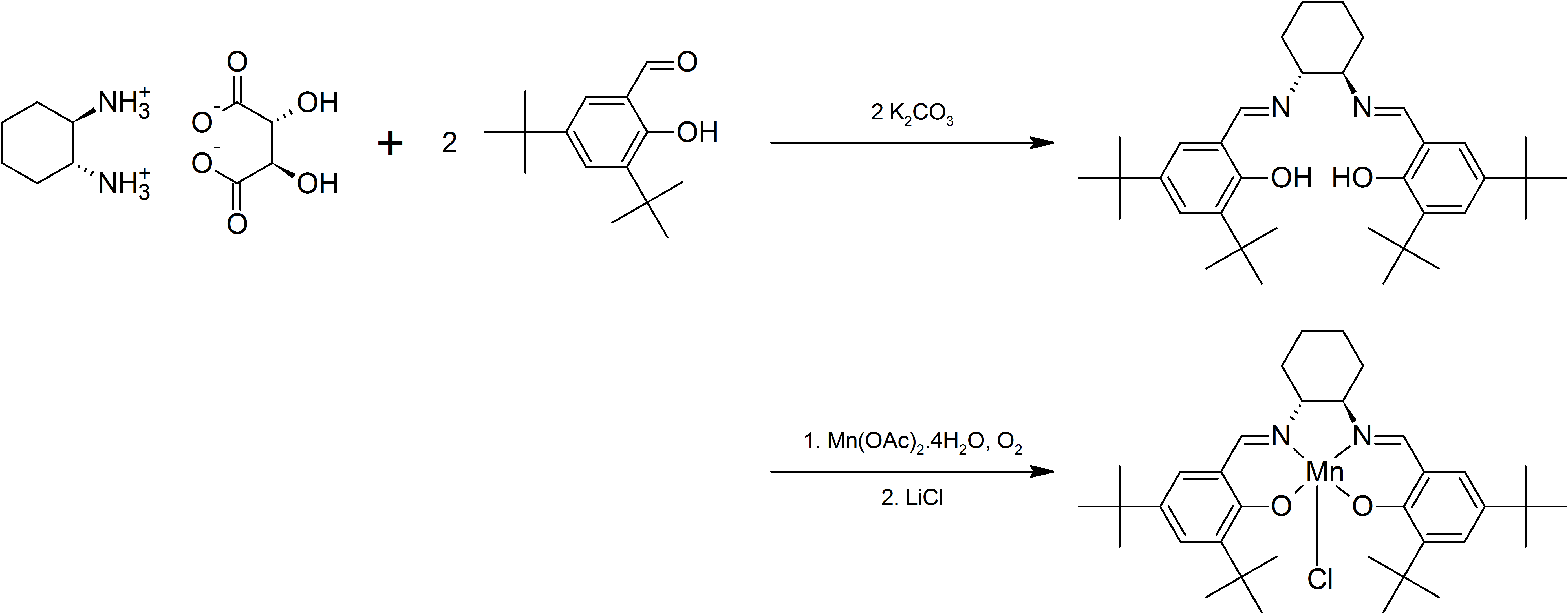
ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elec ...
derived from ''N'',''N''′-bis(salicylidene)ethylenediamine, commonly called salen. The classical example is salcomine, the complex with divalent
In chemistry, the valence (US spelling) or valency (British spelling) of an chemical element, element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
Description
The combining capacity, ...
cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, p ...
, usually denoted as Co(salen). These complexes are widely investigated as catalysts and enzyme mimics.
phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it ...
ic hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydro ...
groups. The salen ligand
Salen refers to a tetradentate C2-symmetric ligand synthesized from salicylaldehyde (sal) and ethylenediamine (en). It may also refer to a class of compounds, which are structurally related to the classical salen ligand, primarily bis-Schiff base ...
is usually its conjugate base (salen2−), resulting from the loss of protons from those hydroxyl groups. The metal atom usually makes four coordination bonds to the oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
and nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
atoms.
Preparation of complexes
The salen anion forms complexes with mosttransition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that ca ...
s. These complexes are usually prepared by the reaction of H2salen ("proligand") with metal precursors containing built-in bases, such as alkoxides, metal amides, or metal acetate
Transition metal carboxylate complexes are coordination complexes with carboxylate (RCO2−) ligands. Reflecting the diversity of carboxylic acids, the inventory of metal carboxylates is large. Many are useful commercially, and many have attracted ...
. The proligand may also be treated with a metal halide
Metal halides are compounds between metals and halogens. Some, such as sodium chloride are ionic, while others are covalently bonded. A few metal halides are discrete molecules, such as uranium hexafluoride, but most adopt polymeric structures, ...
, with or without an added base. Lastly, the proligand may be deprotonated by a nonnucleophilic base, such as sodium hydride
Sodium hydride is the chemical compound with the empirical formula Na H. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in co ...
, before treatment with the metal halide. For example, Jacobsen's catalyst
Jacobsen's catalyst is the common name for N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride, a coordination compound of manganese and a salen-type ligand. It is used as an asymmetric catalyst in the Jacobs ...
is prepared from the salen ligand precursor with manganese acetate.
Structures
square planar molecular geometry
The square planar molecular geometry in chemistry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain chemical compounds. As the name suggests, molecules of this geometry have their atoms positioned at the corn ...
in the coordination sphere
In coordination chemistry, the first coordination sphere refers to the array of molecules and ions (the ligands) directly attached to the central metal atom. The second coordination sphere consists of molecules and ions that attached in various ...
.
Other metal–salen complexes may have additional ligands above the salen nitrogen–oxygen plane. Complexes with one extra ligand, such as VO(salen), may have a square pyramidal molecular geometry
In molecular geometry, square pyramidal geometry describes the shape of certain Chemical compound, compounds with the formula where L is a ligand. If the ligand atoms were connected, the resulting shape would be that of a Square pyramid, pyram ...
. Complexes with two extra ligands, such as Co(salen)Cl( py), may have octahedral geometry
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The oc ...
. Usually the MN2O2 core is relatively planar, even though the ethylene backbone is skewed and the overall salen ligand takes a twisted C2 symmetry. Examples exist where ancillary ligands force the N2O2 donors out of planarity. No evidence indicates that salen is a redox- noninnocent ligand.
Reactions
Enzyme mimics
Tsumaki described the first metal–salen complexes in 1938. He found that the cobalt(II) complex Co(salen) reversibly binds O2, which led to intensive research on cobalt complexes of salen and relatedligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elec ...
s for their capacity for oxygen storage and transport, looking for potential synthetic oxygen carriers. Cobalt salen complexes also replicate certain aspects of vitamin B12.
Homogeneous catalysis
The manganese-containing salen complex catalyzes the asymmetric epoxidation ofalkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s. In the hydrolytic kinetic resolution technique, a racemic
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. ...
mixture of epoxides
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale f ...
may be separated by selectively hydrolyzing
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
one enantiomer, catalyzed by the analogous cobalt(III) complex. In subsequent work, chromium(III) and cobalt(III) salen complexes catalyze the reaction of carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
and epoxides to give polycarbonates
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. They are easily worke ...
.
Related complexes
Substituted salen complexes
organic solvents
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for p ...
. Side chains attached to the ethylene bridge or the benzene rings may increase the solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solub ...
. An example is the salpn ligand, derived from 1,2-diaminopropane instead of ethylenediamine, which is used as a metal deactivating additive in motor oils and motor fuel
An engine or motor is a machine designed to convert one or more forms of energy into mechanical energy.
Available energy sources include potential energy (e.g. energy of the Earth's gravitational field as exploited in hydroelectric power g ...
.
The presence of bulky groups near the coordination site is generally desirable, as it enhances catalytic activity and prevents dimerization. Salen ligands derived from 3,5-di-''tert''-butylsalicylaldehyde are popular because they fulfill both criteria, and tend to be soluble even in non-polar solvents like pentane.
Chirality may be introduced into the ligand either via the diamine backbone, via the phenyl ring, or both. For example, condensation of the C2-symmetric ''trans''-1,2- diaminocyclohexane with 3,5-di-''tert''-butylsalicylaldehdye gives a ligand that forms complexes with Cr, Mn, Co, Al, which have proven useful for asymmetric transformations. For an example, see the Jacobsen epoxidation
The Jacobsen epoxidation, sometimes also referred to as Jacobsen-Katsuki epoxidation is a chemical reaction which allows enantioselective epoxidation of unfunctionalized alkyl- and aryl- substituted alkenes. It is complementary to the Sharpless e ...
, which is catalyzed by a chiral manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy use ...
-salen complex:
:
Complexes with salen-type ligands
"Salen-type" metal complexes are formed with ligands with similar chelating groups, such as acacen, salph, and salqu. Salqu copper complexes have been investigated as oxidation catalysts.Complexes with salan ligands
Complexes with the similarsalan
]
Salan, Salanus or Zalan ( Bulgarian language, Bulgarian and Serbian Cyrillic: Салан or Залан; hu, Zalán; ro, Salanus) was, according to the Gesta Hungarorum, a local Bulgarianhttp://keptar.niif.hu/000500/000586/magyaro-honf-terke ...
or salalen ligand, salalen ligands, with one or two saturated nitrogen–aryl bonds (amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
s rather than imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
s) tend to be less rigid and more electron-rich at the metal center than the corresponding salen complexes.
Further reading
* *References
{{Reflist Coordination chemistry Organometallic chemistry Ligands