|
Square Pyramidal Molecular Geometry
In molecular geometry, square pyramidal geometry describes the shape of certain compounds with the formula where L is a ligand. If the ligand atoms were connected, the resulting shape would be that of a pyramid with a square base. The point group symmetry involved is of type C4v. The geometry is common for certain main group compounds that have a stereochemically-active lone pair, as described by VSEPR theory. Certain compounds crystallize in both the trigonal bipyramidal and the square pyramidal structures, notably . As a transition state in Berry pseudorotation As a trigonal bipyramidal molecule undergoes Berry pseudorotation, it proceeds via an intermediary stage with the square pyramidal geometry. Thus even though the geometry is rarely seen as the ground state, it is accessed by a low energy distortion from a trigonal bipyramid. Pseudorotation also occurs in square pyramidal molecules. Molecules with this geometry, as opposed to trigonal bipyramidal, exhibit heavier ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Symmetry In Three Dimensions
In three dimensional geometry, there are four infinite series of point groups in three dimensions (''n''≥1) with ''n''-fold rotational or reflectional symmetry about one axis (by an angle of 360°/''n'') that does not change the object. They are the finite symmetry groups on a cone. For ''n'' = ∞ they correspond to four frieze groups. Schönflies notation is used. The terms horizontal (h) and vertical (v) imply the existence and direction of reflections with respect to a vertical axis of symmetry. Also shown are Coxeter notation in brackets, and, in parentheses, orbifold notation. Types ;Chiral: *''Cn'', sup>+, (''nn'') of order ''n'' - ''n''-fold rotational symmetry - acro-n-gonal group (abstract group ''Zn''); for ''n''=1: no symmetry (trivial group) ;Achiral: *''Cnh'', +,2 (''n''*) of order 2''n'' - prismatic symmetry or ortho-n-gonal group (abstract group ''Zn'' × ''Dih1''); for ''n''=1 this is denoted by ''Cs'' (1*) and called reflection symmetry, also bilateral ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallize
Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposition directly from a gas. Attributes of the resulting crystal depend largely on factors such as temperature, air pressure, and in the case of liquid crystals, time of fluid evaporation. Crystallization occurs in two major steps. The first is nucleation, the appearance of a crystalline phase from either a supercooled liquid or a supersaturated solvent. The second step is known as crystal growth, which is the increase in the size of particles and leads to a crystal state. An important feature of this step is that loose particles form layers at the crystal's surface and lodge themselves into open inconsistencies such as pores, cracks, etc. The majority of minerals and organic molecules crystallize easily, and the resulting crystals are gener ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypervalent Molecule
In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains one or more main group elements apparently bearing more than eight electrons in their valence shells. Phosphorus pentachloride (), sulfur hexafluoride (), chlorine trifluoride (), the chlorite () ion, and the triiodide () ion are examples of hypervalent molecules. Definitions and nomenclature Hypervalent molecules were first formally defined by Jeremy I. Musher in 1969 as molecules having central atoms of group 15–18 in any valence other than the lowest (i.e. 3, 2, 1, 0 for Groups 15, 16, 17, 18 respectively, based on the octet rule). Several specific classes of hypervalent molecules exist: * Hypervalent iodine compounds are useful reagents in organic chemistry (e.g. Dess–Martin periodinane) * Tetra-, penta- and hexavalent phosphorus, silicon, and sulfur compounds (e.g. PCl5, PF5, SF6, sulfuranes and persulfuranes) * Noble gas compounds (ex. xe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AXE Method
Valence shell electron pair repulsion (VSEPR) theory ( , ), is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Nyholm. The premise of VSEPR is that the valence electron pairs surrounding an atom tend to repel each other and will, therefore, adopt an arrangement that minimizes this repulsion. This in turn decreases the molecule's energy and increases its stability, which determines the molecular geometry. Gillespie has emphasized that the electron-electron repulsion due to the Pauli exclusion principle is more important in determining molecular geometry than the electrostatic repulsion. The insights of VSEPR theory are derived from topological analysis of the electron density of molecules. Such quantum chemical topology (QCT) methods include the electron localization function (ELF) and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylacetone
Acetylacetone is an organic compound with the chemical formula . It is a colorless liquid, classified as a 1,3-diketone. It exists in equilibrium with a tautomer . These tautomers interconvert so rapidly under most conditions that they are treated as a single compound in most applications. It is a colorless liquid that is a precursor to acetylacetonate anion (commonly abbreviated acac−), a bidentate ligand. It is also a building block for the synthesis of heterocyclic compounds. Properties Tautomerism The keto and enol tautomers of acetylacetone coexist in solution. The enol form has C2v symmetry, meaning the hydrogen atom is shared equally between the two oxygen atoms. In the gas phase, the equilibrium constant, ''K''keto→enol, is 11.7, favoring the enol form. The two tautomeric forms can be distinguished by NMR spectroscopy, IR spectroscopy and other methods. The equilibrium constant tends to be high in nonpolar solvents; when k = >1, the enol form is favour ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadyl Acetylacetonate
Vanadyl acetylacetonate is the chemical compound with the formula VO(acac)2, where acac– is the conjugate base of acetylacetone. It is a blue-green solid that dissolves in polar organic solvents. The coordination complex consists of the vanadyl group, VO2+, bound to two acac– ligands via the two oxygen atoms on each. Like other charge-neutral acetylacetonate complexes, it is not soluble in water. Synthesis The complex is generally prepared from vanadium(IV), e.g. vanadyl sulfate: :VOSO4 + 2 Hacac → VO(acac)2 + H2SO4 It can also be prepared by a redox reaction starting with vanadium pentoxide. In this reaction, some acetylacetone is oxidized to acetic anhydride. Structure and properties The complex has a square pyramidal structure with a short V=O bond. This d1 compound is paramagnetic. Its optical spectrum exhibits two transitions. It is a weak Lewis acid, forming adducts with pyridine and methylamine. Applications It is used in organic chemistry as a catalyst for th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer ( passivation) somewhat stabilizes the free metal against further oxidation. Spanish scientist Andrés Manuel del Río discovered compounds of vanadium in 1801 in Mexico by analyzing a new lead-bearing mineral he called "brown lead". Though he initially presumed its qualities were due to the presence of a new element, he was later erroneously convinced by French chemist Hippolyte Victor Collet-Descotils that the element was just chromium. Then in 1830, Nils Gabriel Sefström generated chlorides of vanadium, thus proving there was a new element, and named it "vanadium" after the Scandinavian goddess of beauty and fertility, Vanadís (Freyja). The name was based on the wide range of colors found in vanadium compounds. Del Rio's lead mineral was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is known as group 17. The word "halogen" means "salt former" (or "salt maker"). When halogens react with metals, they produce a wide range of salts, including calcium fluoride, sodium chloride (common table salt), silver bromide and potassium iodide. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure. All of the halogens form acids when bonded to hydrogen. Most halogens are typically produced from minerals or salts. The middle halogens—chlorine, bromine, and iodine—are often used as disinfectants. Organobromides are the most important class of flame retardants, while elemental halogens are dangerous and can be toxic. History The fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berry Pseudorotation
The Berry mechanism, or Berry pseudorotation mechanism, is a type of vibration causing molecules of certain geometries to isomerize by exchanging the two axial ligands (see Figure at right) for two of the equatorial ones. It is the most widely accepted mechanism for pseudorotation and most commonly occurs in trigonal bipyramidal molecules such as PF5, though it can also occur in molecules with a square pyramidal geometry. The Berry mechanism is named after R. Stephen Berry, who first described this mechanism in 1960.RS Berry, 1960, "Correlation of rates of intramolecular tunneling processes, with application to some Group V compounds," ''J. Chem. Phys.'' 32:933-938, DOI 10.1063/1.1730820; seo accessed 28 May 2014M Cass, KK Hii & HS Rzepa, 2005, "Mechanisms that interchange axial and equatorial atoms in fluxional processes: Illustration of the Berry pseudorotation, the turnstile and the lever mechanisms via animation of transition state normal vibrational modes", ''J. Chem. Educ. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
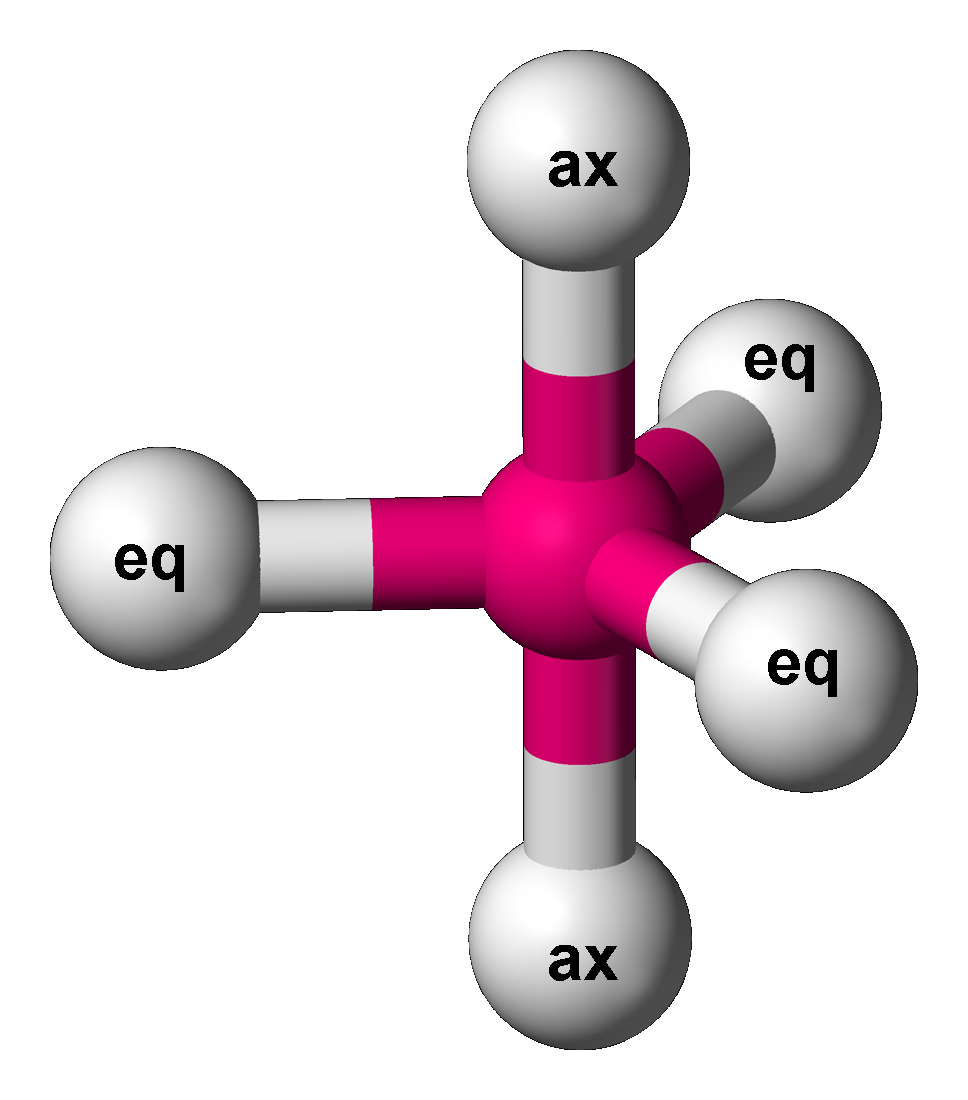
Trigonal Bipyramidal
In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. This is one geometry for which the bond angles surrounding the central atom are not identical (see also pentagonal bipyramid), because there is no geometrical arrangement with five terminal atoms in equivalent positions. Examples of this molecular geometry are phosphorus pentafluoride (), and phosphorus pentachloride () in the gas phase. Axial (or apical) and equatorial positions The five atoms bonded to the central atom are not all equivalent, and two different types of position are defined. For phosphorus pentachloride as an example, the phosphorus atom shares a plane with three chlorine atoms at 120° angles to each other in ''equatorial'' positions, and two more chlorine atoms above and below the plane (''axial'' or ''apical'' positions). According to the VSEPR theory of molecular geometry, an axial position is more crowd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
VSEPR Theory
Valence shell electron pair repulsion (VSEPR) theory ( , ), is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Nyholm. The premise of VSEPR is that the valence electron pairs surrounding an atom tend to repel each other and will, therefore, adopt an arrangement that minimizes this repulsion. This in turn decreases the molecule's energy and increases its stability, which determines the molecular geometry. Gillespie has emphasized that the electron-electron repulsion due to the Pauli exclusion principle is more important in determining molecular geometry than the electrostatic repulsion. The insights of VSEPR theory are derived from topological analysis of the electron density of molecules. Such quantum chemical topology (QCT) methods include the electron localization function (ELF) and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |