Rotaxane on:
[Wikipedia]
[Google]
[Amazon]
 In
In
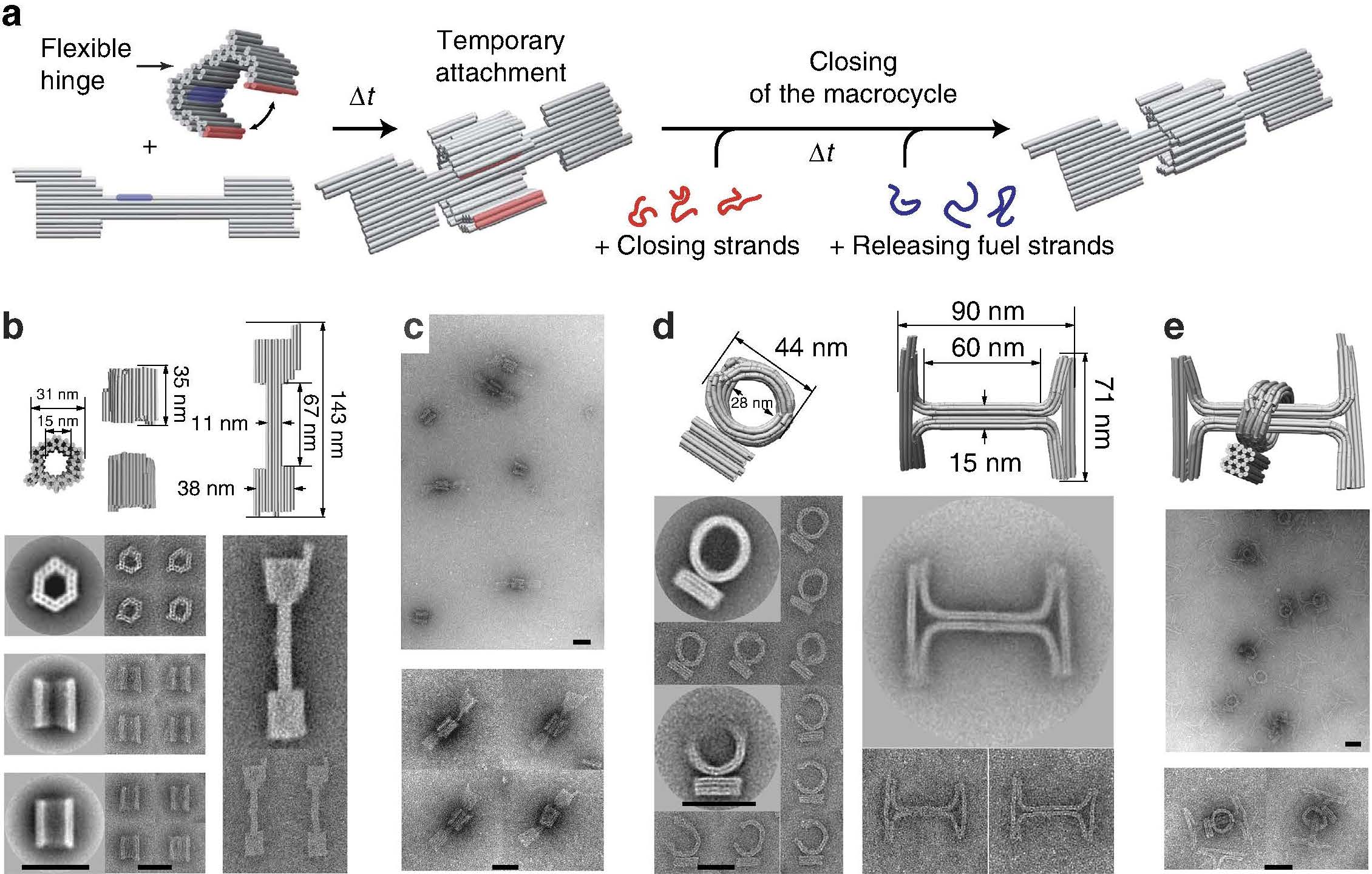
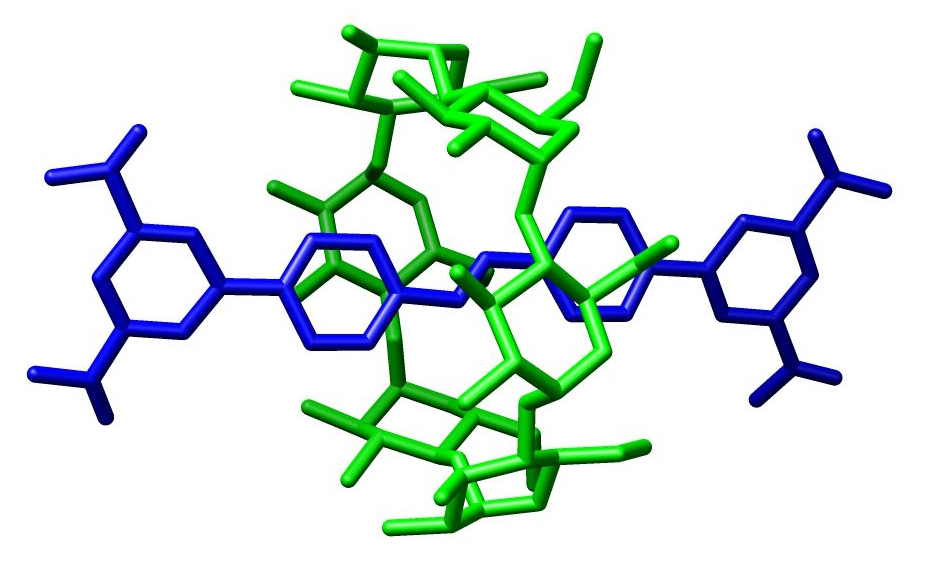
 In
In chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, proper ...
, a rotaxane () is a mechanically interlocked molecular architecture consisting of a dumbbell
The dumbbell, a type of free weight, is a piece of equipment used in weight training. It can be used individually or in pairs, with one in each hand.
History
The forerunner of the dumbbell, halteres, were used in ancient Greece as lifting ...
-shaped molecule which is threaded through a macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.
...
(see graphical representation). The two components of a rotaxane are kinetically trapped since the ends of the dumbbell (often called ''stoppers'') are larger than the internal diameter of the ring and prevent dissociation (unthreading) of the components since this would require significant distortion of the covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between ato ...
s.
Much of the research concerning rotaxanes and other mechanically interlocked molecular architectures, such as catenane
In macromolecular chemistry, a catenane () is a mechanically interlocked molecular architecture consisting of two or more interlocked macrocycles, i.e. a molecule containing two or more intertwined rings. The interlocked rings cannot be se ...
s, has been focused on their efficient synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
* Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
**Organic synthesis, the chemical synthesis of organ ...
or their utilization as artificial molecular machine
A molecular machine, nanite, or nanomachine is a molecular component that produces quasi-mechanical movements (output) in response to specific stimuli (input). In cellular biology, macromolecular machines frequently perform tasks essential for l ...
s. However, examples of rotaxane substructure have been found in naturally occurring peptides
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
...
, including: cystine knot
A cystine knot is a protein structural motif containing three disulfide bridges (formed from pairs of cysteine residues). The sections of polypeptide that occur between two of them form a loop through which a third disulfide bond passes, forming ...
peptides, cyclotide
In biochemistry, cyclotides are small, disulfide-rich peptides isolated from plants. Typically containing 28-37 amino acids, they are characterized by their head-to-tail cyclised peptide backbone and the interlocking arrangement of their three ...
s or lasso-peptides such as microcin J25.
Synthesis
The earliest reported synthesis of a rotaxane in 1967 relied on the statistical probability that if two halves of a dumbbell-shaped molecule were reacted in the presence of amacrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.
...
that some small percentage would connect through the ring. To obtain a reasonable quantity of rotaxane, the macrocycle was attached to a solid-phase support and treated with both halves of the dumbbell 70 times and then severed from the support to give a 6% yield. However, the synthesis of rotaxanes has advanced significantly and efficient yields can be obtained by preorganizing the components utilizing hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
ing, metal coordination, hydrophobic forces, covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between ato ...
s, or coulombic interactions. The three most common strategies to synthesize rotaxane are "capping", "clipping", and "slipping", though others do exist. Recently, Leigh and co-workers described a new pathway to mechanically interlocked architectures involving a transition-metal center that can catalyse a reaction through the cavity of a macrocycle.

Capping
Synthesis via the capping method relies strongly upon a thermodynamically driven template effect; that is, the "thread" is held within the "macrocycle" by non-covalent interactions, for example rotaxinations with cyclodextrin macrocycles involve exploitation of the hydrophobic effect. This dynamic complex or pseudorotaxane is then converted to the rotaxane by reacting the ends of the threaded guest with large groups, preventing disassociation.Clipping
The clipping method is similar to the capping reaction except that in this case the dumbbell shaped molecule is complete and is bound to a partial macrocycle. The partial macrocycle then undergoes a ring closing reaction around the dumbbell-shaped molecule, forming the rotaxane.Slipping
The method of slipping is one which exploits the thermodynamic stability of the rotaxane. If the end groups of the dumbbell are an appropriate size it will be able to reversibly thread through the macrocycle at higher temperatures. By cooling the dynamic complex, it becomes kinetically trapped as a rotaxane at the lower temperature."Active template" methodology
Leigh and co-workers recently began to explore a strategy in which template ions could also play an active role in promoting the crucial final covalent bond forming reaction that captures the interlocked structure (i.e., the metal has a dual function, acting as a template for entwining the precursors and catalyzing covalent bond formation between the reactants).Potential applications

Molecular machines
Rotaxane-based molecular machines have been of initial interest for their potential use inmolecular electronics
Molecular electronics is the study and application of molecular building blocks for the fabrication of electronic components. It is an interdisciplinary area that spans physics, chemistry, and materials science. The unifying feature is use of mo ...
as logic molecular switch
A molecular switch is a molecule that can be reversibly shifted between two or more stable states. The molecules may be shifted between the states in response to environmental stimuli, such as changes in pH, light, temperature, an electric curren ...
ing elements and as molecular shuttle
A molecular shuttle in supramolecular chemistry is a special type of molecular machine capable of shuttling molecules or ions from one location to another. This field is of relevance to nanotechnology in its quest for nanoscale electronic compone ...
s. These molecular machines are usually based on the movement of the macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.
...
on the dumbbell. The macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.
...
can rotate around the axis of the dumbbell like a wheel and axle or it can slide along its axis from one site to another. Controlling the position of the macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.
...
allows the rotaxane to function as a molecular switch, with each possible location of the macrocycle corresponding to a different state. These rotaxane machines can be manipulated both by chemical and photochemical inputs. Rotaxane based systems have also been shown to function as molecular muscles. In 2009, there was a report of a "domino effect" from one extremity to the other in a Glycorotaxane Molecular Machine. In this case, the 4''C''1 or 1''C''4 chair-like conformation of the manno pyranoside stopper can be controlled, depending on the localization of the macrocycle. In 2012, unique pseudo-macrocycles consisting of double-lasso molecular machines (also called rotamacrocycles) were reported in Chem. Sci. These structures can be tightened or loosened depending on pH. A controllable jump rope movement was also observed in these new molecular machines.
Ultrastable dyes
Potential application as long-lasting dyes is based on the enhanced stability of the inner portion of the dumbbell-shaped molecule. Studies withcyclodextrin
Cyclodextrins are a family of cyclic oligosaccharides, consisting of a macrocyclic ring of glucose subunits joined by α-1,4 glycosidic bonds. Cyclodextrins are produced from starch by enzymatic conversion. They are used in food, pharmaceutical ...
-protected rotaxane azo dye
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C-N ...
s established this characteristic. More reactive squaraine dyes have also been shown to have enhanced stability by preventing nucleophilic attack of the inner squaraine moiety
Moiety may refer to:
Chemistry
* Moiety (chemistry), a part or functional group of a molecule
** Moiety conservation, conservation of a subgroup in a chemical species
Anthropology
* Moiety (kinship), either of two groups into which a society is ...
. The enhanced stability of rotaxane dyes is attributed to the insulating effect of the macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.
...
, which is able to block interactions with other molecules.
Nanorecording
In a nanorecording application, a certain rotaxane is deposited as aLangmuir–Blodgett film
A Langmuir–Blodgett (LB) film is a nanostructured system formed when Langmuir films—or Langmuir monolayers (LM)—are transferred from the liquid-gas interface to solid supports during the vertical passage of the support through the monolayers ...
on ITO-coated glass. When a positive voltage
Voltage, also known as electric pressure, electric tension, or (electric) potential difference, is the difference in electric potential between two points. In a static electric field, it corresponds to the work needed per unit of charge to ...
is applied with the tip of a scanning tunneling microscope
A scanning tunneling microscope (STM) is a type of microscope used for imaging surfaces at the atomic level. Its development in 1981 earned its inventors, Gerd Binnig and Heinrich Rohrer, then at IBM Zürich, the Nobel Prize in Physics in 1986 ...
probe, the rotaxane rings in the tip area switch to a different part of the dumbbell and the resulting new conformation makes the molecules stick out 0.3 nanometer
330px, Different lengths as in respect to the molecular scale.
The nanometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: nm) or nanometer (American and British English spelling differences#-re, ...
from the surface. This height difference is sufficient for a memory dot. It is not yet known how to erase such a nanorecording film.
Nomenclature
Accepted nomenclature is to designate the number of components of the rotaxane in brackets as a prefix. Therefore, the a rotaxane consisting of a single dumbbell-shaped axial molecule with a single macrocycle around its shaft is called a otaxane, and two cyanostar molecules around the central phosphate group of dialkylphosphate is a otaxane.See also
*Catenane
In macromolecular chemistry, a catenane () is a mechanically interlocked molecular architecture consisting of two or more interlocked macrocycles, i.e. a molecule containing two or more intertwined rings. The interlocked rings cannot be se ...
* Mechanically interlocked molecular architecture
*Molecular Borromean rings
In chemistry, molecular Borromean rings are an example of a mechanically-interlocked molecular architecture in which three macrocycles are interlocked in such a way that breaking any macrocycle allows the others to dissociate. They are the smal ...
* Molecular knots
* Polyrotaxane
References
{{reflist, 30em Supramolecular chemistry Molecular electronics Nanoelectronics Organic semiconductors Molecular topology Articles containing video clips