The 1918ŌĆō1920 influenza pandemic, commonly known by the
misnomer
A misnomer is a name that is incorrectly or unsuitably applied. Misnomers often arise because something was named long before its correct nature was known, or because an earlier form of something has been replaced by a later form to which the name ...
Spanish flu or as the Great Influenza epidemic, was an exceptionally deadly global
influenza pandemic
An influenza pandemic is an epidemic of an influenza virus that spreads across a large region (either multiple continents or worldwide) and infects a large proportion of the population. There have been six major influenza epidemics in the last ...
caused by the
H1N1 influenza A virus. The earliest documented case was March 1918 in
Kansas
Kansas () is a state in the Midwestern United States. Its capital is Topeka, and its largest city is Wichita. Kansas is a landlocked state bordered by Nebraska to the north; Missouri to the east; Oklahoma to the south; and Colorado to the ...
, United States, with further cases recorded in France, Germany and the United Kingdom in April. Two years later, nearly a third of the global population, or an estimated 500 million people, had been infected in four successive waves. Estimates of deaths range from 17 million to 50 million, and possibly as high as 100 million, making it
one of the deadliest pandemics in history.
The pandemic broke out near the end of
World War I
World War I (28 July 1914 11 November 1918), often abbreviated as WWI, was one of the deadliest global conflicts in history. Belligerents included much of Europe, the Russian Empire, the United States, and the Ottoman Empire, with fightin ...
, when wartime
censors
Censorship is the suppression of speech, public communication, or other information. This may be done on the basis that such material is considered objectionable, harmful, sensitive, or "inconvenient". Censorship can be conducted by governments ...
suppressed bad news in the
belligerent
A belligerent is an individual, group, country, or other entity that acts in a hostile manner, such as engaging in combat. The term comes from the Latin ''bellum gerere'' ("to wage war"). Unlike the use of ''belligerent'' as an adjective meaning ...
countries to maintain
morale
Morale, also known as esprit de corps (), is the capacity of a group's members to maintain belief in an institution or goal, particularly in the face of opposition or hardship. Morale is often referenced by authority figures as a generic value ...
, but newspapers
freely reported the outbreak in
neutral Spain, creating a false impression of Spain as the epicenter and leading to the "Spanish flu" misnomer.
[ Limited historical ]epidemiological
Epidemiology is the study and analysis of the distribution (who, when, and where), patterns and determinants of health and disease conditions in a defined population.
It is a cornerstone of public health, and shapes policy decisions and evidenc ...
data make the pandemic's geographic origin indeterminate, with competing hypotheses on the initial spread.
Most influenza outbreaks disproportionately kill the young and old, with a higher survival rate in-between, but this pandemic had unusually high mortality for young adults. Scientists offer several explanations for the high mortality, including a six-year climate anomaly affecting migration of disease vectors with increased likelihood of spread through bodies of water.cytokine storm
A cytokine storm, also called hypercytokinemia, is a physiological reaction in humans and other animals in which the innate immune system causes an uncontrolled and excessive release of pro-inflammatory signaling molecules called cytokines. Norm ...
, ravaging the stronger immune system
The immune system is a network of biological processes that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as cancer cells and objects such as wood splinte ...
of young adults, although the viral infection
A viral disease (or viral infection) occurs when an organism's body is invaded by pathogenic viruses, and infectious virus particles (virions) attach to and enter susceptible cells.
Structural Characteristics
Basic structural characteristics, s ...
was apparently no more aggressive than previous influenza
Influenza, commonly known as "the flu", is an infectious disease caused by influenza viruses. Symptoms range from mild to severe and often include fever, runny nose, sore throat, muscle pain, headache, coughing, and fatigue. These symptoms ...
strains. Malnourishment
Malnutrition occurs when an organism gets too few or too many nutrients, resulting in health problems. Specifically, it is "a deficiency, excess, or imbalance of energy, protein and other nutrients" which adversely affects the body's tissues a ...
, overcrowded medical camps and hospitals, and poor hygiene
Hygiene is a series of practices performed to preserve health.
According to the World Health Organization (WHO), "Hygiene refers to conditions and practices that help to maintain health and prevent the spread of diseases." Personal hygiene refer ...
, exacerbated by the war, promoted bacterial superinfection
A superinfection is a second infection superimposed on an earlier one, especially by a different microbial agent of exogenous or endogenous origin, that is resistant to the treatment being used against the first infection. Examples of this in bact ...
, killing most of the victims after a typically prolonged death bed.2009 swine flu pandemic
The 2009 swine flu pandemic, caused by the H1N1 influenza virus and declared by the World Health Organization (WHO) from June 2009 to August 2010, is the third recent flu pandemic involving the H1N1 virus (the first being the 1918ŌĆō1920 Span ...
.1977 Russian flu
The 1977 Russian flu was an influenza pandemic that was first reported by the Soviet Union in 1977 and lasted until 1979. The outbreak in northern China started in May 1977, slightly earlier than that in the Soviet Union. The pandemic mostly affe ...
was also caused by H1N1 virus.
Etymologies
 This pandemic was known by many different namesŌĆösome old, some newŌĆödepending on place, time, and context. The
This pandemic was known by many different namesŌĆösome old, some newŌĆödepending on place, time, and context. The etymology
Etymology ()The New Oxford Dictionary of English (1998) ŌĆō p. 633 "Etymology /╦ī╔øt╔¬╦łm╔Æl╔Öd╩Æi/ the study of the class in words and the way their meanings have changed throughout time". is the study of the history of the Phonological chan ...
of alternative names historicises the scourge and its effects on people who would only learn years later
Later may refer to:
* Future, the time after the present
Television
* ''Later'' (talk show), a 1988ŌĆō2001 American talk show
* '' Later... with Jools Holland'', a British music programme since 1992
* ''The Life and Times of Eddie Roberts'', or ...
that invisible virus
A virus is a submicroscopic infectious agent that replicates only inside the living cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and archaea.
Since Dmitri Ivanovsky's 1 ...
es caused influenza
Influenza, commonly known as "the flu", is an infectious disease caused by influenza viruses. Symptoms range from mild to severe and often include fever, runny nose, sore throat, muscle pain, headache, coughing, and fatigue. These symptoms ...
. This lack of scientific answers led the ''Sierra Leone Weekly News'' (Freetown
Freetown is the capital and largest city of Sierra Leone. It is a major port city on the Atlantic Ocean and is located in the Western Area of the country. Freetown is Sierra Leone's major urban, economic, financial, cultural, educational and p ...
) to suggest a biblical
The Bible (from Koine Greek , , 'the books') is a collection of religious texts or scriptures that are held to be sacred in Christianity, Judaism, Samaritanism, and many other religions. The Bible is an anthologya compilation of texts of a ...
framing in July 1918, using an interrogative
An interrogative clause is a clause whose form is typically associated with question-like meanings. For instance, the English sentence "Is Hannah sick?" has interrogative syntax which distinguishes it from its declarative counterpart "Hannah is ...
from Exodus 16
Beshalach, Beshallach, or Beshalah (ŌĆöHebrew for "when elet go" (literally: "in (having) sent"), the second word and first distinctive word in the parashah) is the sixteenth weekly Torah portion (, ''parashah'') in the annual Jewish cycle of To ...
in ancient Hebrew
Hebrew (; ; ) is a Northwest Semitic language of the Afroasiatic language family. Historically, it is one of the spoken languages of the Israelites and their longest-surviving descendants, the Jews and Samaritans. It was largely preserved ...
: "One thing is for certainŌĆöthe doctors are at present flabbergasted; and we suggest that rather than calling the disease influenza they should for the present until they have it in hand, say ŌĆö'What is it?'"
Descriptive names
Outbreaks of influenza-like illness
Influenza-like illness (ILI), also known as flu-like syndrome or flu-like symptoms, is a medical diagnosis of possible influenza or other illness causing a set of common symptoms. These include fever, shivering, chills, malaise, dry cough, lo ...
were documented in 1916ŌĆō17 at British military hospitals in ├ētaples
├ētaples or ├ētaples-sur-Mer (; vls, Stapel, lang; pcd, ├ētape) is a commune in the Pas-de-Calais department in northern France. It is a fishing and leisure port on the Canche river.
History
├ētaples takes its name from having been a medieval ...
, France
France (), officially the French Republic ( ), is a country primarily located in Western Europe. It also comprises of Overseas France, overseas regions and territories in the Americas and the Atlantic Ocean, Atlantic, Pacific Ocean, Pac ...
, and just across the English Channel
The English Channel, "The Sleeve"; nrf, la Maunche, "The Sleeve" (Cotentinais) or ( J├©rriais), (Guern├®siais), "The Channel"; br, Mor Breizh, "Sea of Brittany"; cy, M├┤r Udd, "Lord's Sea"; kw, Mor Bretannek, "British Sea"; nl, Het Kana ...
at Aldershot
Aldershot () is a town in Hampshire, England. It lies on heathland in the extreme northeast corner of the county, southwest of London. The area is administered by Rushmoor Borough Council. The town has a population of 37,131, while the Alders ...
, England
England is a country that is part of the United Kingdom. It shares land borders with Wales to its west and Scotland to its north. The Irish Sea lies northwest and the Celtic Sea to the southwest. It is separated from continental Europe b ...
. Clinical indications in common with the 1918 pandemic included rapid symptom progression to a "dusky" heliotrope cyanosis
Cyanosis is the change of body tissue color to a bluish-purple hue as a result of having decreased amounts of oxygen bound to the hemoglobin in the red blood cells of the capillary bed. Body tissues that show cyanosis are usually in locations ...
of the face. This characteristic blue-violet cyanosis in expiring patients led to the name 'purple death'.
The Aldershot physicians later wrote in ''The Lancet
''The Lancet'' is a weekly peer-reviewed general medical journal and one of the oldest of its kind. It is also the world's highest-impact academic journal. It was founded in England in 1823.
The journal publishes original research articles, ...
'', "the influenza pneumococcal purulent bronchitis we and others described in 1916 and 1917 is fundamentally the same condition as the influenza of this present pandemic."Purulent
Pus is an exudate, typically white-yellow, yellow, or yellow-brown, formed at the site of inflammation during bacterial or fungal infection. An accumulation of pus in an enclosed tissue space is known as an abscess, whereas a visible collect ...
bronchitis
Bronchitis is inflammation of the bronchi (large and medium-sized airways) in the lungs that causes coughing. Bronchitis usually begins as an infection in the nose, ears, throat, or sinuses. The infection then makes its way down to the bronchi. ...
' is not yet linked to the same A/H1N1
In virology, influenza A virus subtype H1N1 (A/H1N1) is a subtype of influenza A virus. Major outbreaks of H1N1 strains in humans include the Spanish flu, the 1977 Russian flu pandemic and the 2009 swine flu pandemic. It is an orthomyxovirus ...
virus,[ but it may be a precursor.][
In 1918, ']epidemic
An epidemic (from Ancient Greek, Greek ß╝ÉŽĆ╬» ''epi'' "upon or above" and ╬┤ß┐å╬╝╬┐Žé ''demos'' "people") is the rapid spread of disease to a large number of patients among a given population within an area in a short period of time.
Epidemics ...
influenza
Influenza, commonly known as "the flu", is an infectious disease caused by influenza viruses. Symptoms range from mild to severe and often include fever, runny nose, sore throat, muscle pain, headache, coughing, and fatigue. These symptoms ...
' ( it, influenza, influence),Kansas
Kansas () is a state in the Midwestern United States. Its capital is Topeka, and its largest city is Wichita. Kansas is a landlocked state bordered by Nebraska to the north; Missouri to the east; Oklahoma to the south; and Colorado to the ...
in the U.S.
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country primarily located in North America. It consists of 50 states, a federal district, five major unincorporated territori ...
during late spring, and early reports from Spain
, image_flag = Bandera de Espa├▒a.svg
, image_coat = Escudo de Espa├▒a (mazonado).svg
, national_motto = ''Plus ultra'' (Latin)(English: "Further Beyond")
, national_anthem = (English: "Royal March")
, i ...
began appearing on 21 May. Reports from both places called it 'three-day fever' ().
Associative names
 Many alternative names are
Many alternative names are exonyms
An endonym (from Greek: , 'inner' + , 'name'; also known as autonym) is a common, ''native'' name for a geographical place, group of people, individual person, language or dialect, meaning that it is used inside that particular place, group, o ...
in the practice of making new infectious diseases
An infection is the invasion of tissues by pathogens, their multiplication, and the reaction of host tissues to the infectious agent and the toxins they produce. An infectious disease, also known as a transmissible disease or communicable dise ...
seem foreign.epithet
An epithet (, ), also byname, is a descriptive term (word or phrase) known for accompanying or occurring in place of a name and having entered common usage. It has various shades of meaning when applied to seemingly real or fictitious people, di ...
s were re-used in the 1918 pandemic, along with new ones.[
]
'Spanish' influenza
 Outside Spain, the disease was soon misnamed 'Spanish influenza'. In a 2 June 1918 ''
Outside Spain, the disease was soon misnamed 'Spanish influenza'. In a 2 June 1918 ''The Times
''The Times'' is a British daily national newspaper based in London. It began in 1785 under the title ''The Daily Universal Register'', adopting its current name on 1 January 1788. ''The Times'' and its sister paper ''The Sunday Times'' (fou ...
'' of London
London is the capital and largest city of England and the United Kingdom, with a population of just under 9 million. It stands on the River Thames in south-east England at the head of a estuary down to the North Sea, and has been a majo ...
dispatch titled, "The Spanish Epidemic," a correspondent in Madrid
Madrid ( , ) is the capital and most populous city of Spain. The city has almost 3.4 million inhabitants and a metropolitan area population of approximately 6.7 million. It is the second-largest city in the European Union (EU), and ...
reported over 100,000 victims of, "The unknown diseaseŌĆ”clearly of a gripal character," without referring to "Spanish influenza" directly. Three weeks later ''The Times'' reported that, "Everybody thinks of it as the 'Spanish' influenza to-day." Three days after that an advertisement appeared in ''The Times'' for Formamint tablets to prevent "Spanish influenza". When it reached Moscow, ''Pravda
''Pravda'' ( rus, ą¤čĆą░ą▓ą┤ą░, p=╦łpravd╔Ö, a=Ru-ą┐čĆą░ą▓ą┤ą░.ogg, "Truth") is a Russian broadsheet newspaper, and was the official newspaper of the Communist Party of the Soviet Union, when it was one of the most influential papers in the co ...
'' announced, " (the Spanish lady) is in town," making 'the Spanish lady' another common name.
The outbreak did not originate in Spain (see below
Below may refer to:
*Earth
*Ground (disambiguation)
*Soil
*Floor
*Bottom (disambiguation)
Bottom may refer to:
Anatomy and sex
* Bottom (BDSM), the partner in a BDSM who takes the passive, receiving, or obedient role, to that of the top or ...
),censorship
Censorship is the suppression of speech, public communication, or other information. This may be done on the basis that such material is considered objectionable, harmful, sensitive, or "inconvenient". Censorship can be conducted by governments ...
in belligerent
A belligerent is an individual, group, country, or other entity that acts in a hostile manner, such as engaging in combat. The term comes from the Latin ''bellum gerere'' ("to wage war"). Unlike the use of ''belligerent'' as an adjective meaning ...
nations. Spain was a neutral country
A neutral country is a state that is neutral towards belligerents in a specific war or holds itself as permanently neutral in all future conflicts (including avoiding entering into military alliances such as NATO, CSTO or the SCO). As a type of ...
unconcerned with appearances of combat readiness
readiness is a condition of the armed forces and their constituent units and formations, warships, aircraft, weapon systems or other military technology and equipment to perform during combat military operations, or functions consistent with the ...
, and without a wartime propaganda
Propaganda is communication that is primarily used to Social influence, influence or persuade an audience to further an Political agenda, agenda, which may not be Objectivity (journalism), objective and may be selectively presenting facts to en ...
machine to prop up morale
Morale, also known as esprit de corps (), is the capacity of a group's members to maintain belief in an institution or goal, particularly in the face of opposition or hardship. Morale is often referenced by authority figures as a generic value ...
; so its newspapers freely reported epidemic effects, including King Alfonso XIII
Alfonso XIII (17 May 1886 ŌĆō 28 February 1941), also known as El Africano or the African, was King of Spain from 17 May 1886 to 14 April 1931, when the Second Spanish Republic was proclaimed. He was a monarch from birth as his father, Alfo ...
's illness, making Spain the apparent locus of the epidemic. The censorship was so effective that Spain's health officials were unaware its neighboring countries were similarly affected.Journal of the American Medical Association
''The Journal of the American Medical Association'' (''JAMA'') is a peer-reviewed medical journal published 48 times a year by the American Medical Association. It publishes original research, reviews, and editorials covering all aspects of bio ...
'', a Spanish official protested, "we were surprised to learn that the disease was making ravages in other countries, and that people there were calling it the 'Spanish grip'. And wherefore Spanish? ŌĆ”this epidemic was not born in Spain, and this should be recorded as a historic vindication." But before this letter could be published, ''The Serbian Newspaper'' (Corfu
Corfu (, ) or Kerkyra ( el, ╬Ü╬ŁŽü╬║ŽģŽü╬▒, K├®rkyra, , ; ; la, Corcyra.) is a Greek island in the Ionian Sea, of the Ionian Islands, and, including its small satellite islands, forms the margin of the northwestern frontier of Greece. The isl ...
) said, "Various countries have been assigning the origin of this imposing guest to each other for quite some time, and at one point in time they agreed to assign its origin to the kind and neutral SpainŌĆ”"
Other exonyms
French press initially used 'American flu', but adopted 'Spanish flu' in lieu of antagonizing an ally.battlefield
A battlefield, battleground, or field of battle is the location of a present or historic battle involving ground warfare. It is commonly understood to be limited to the point of contact between opposing forces, though battles may involve troops ...
in Belgium
Belgium, ; french: Belgique ; german: Belgien officially the Kingdom of Belgium, is a country in Northwestern Europe. The country is bordered by the Netherlands to the north, Germany to the east, Luxembourg to the southeast, France to th ...
where many soldiers on both sides fell ill.Senegal
Senegal,; Wolof: ''Senegaal''; Pulaar: ׿ģ׿½×ż▓׿½×ż║׿óןä׿ż×żŁ (Senegaali); Arabic: ž¦┘äž│┘åž║ž¦┘ä ''As-Sinighal'') officially the Republic of Senegal,; Wolof: ''R├®ewum Senegaal''; Pulaar : ׿ł×ż½×ż▓׿Ż×żóןä׿▓׿Ż×żŁ × ...
it was named 'Brazilian flu', and in Brazil
Brazil ( pt, Brasil; ), officially the Federative Republic of Brazil (Portuguese: ), is the largest country in both South America and Latin America. At and with over 217 million people, Brazil is the world's fifth-largest country by area ...
, 'German flu'. In Spain it was also known as the 'French flu' (),zarzuela
() is a Spanish lyric-dramatic genre that alternates between spoken and sung scenes, the latter incorporating operatic and popular songs, as well as dance. The etymology of the name is uncertain, but some propose it may derive from the name of ...
.[ Spanish flu () is now a common name in Spain, but remains controversial there.]Bolshevik
The Bolsheviks (russian: ąæąŠą╗čīčłąĄą▓ąĖą║ąĖ╠ü, from ą▒ąŠą╗čīčłąĖąĮčüčéą▓ąŠ╠ü ''bol'shinstv├│'', 'majority'),; derived from ''bol'shinstv├│'' (ą▒ąŠą╗čīčłąĖąĮčüčéą▓ąŠ╠ü), "majority", literally meaning "one of the majority". also known in English ...
disease',[ Some Africans called it a 'white man's sickness', but in ]South Africa
South Africa, officially the Republic of South Africa (RSA), is the southernmost country in Africa. It is bounded to the south by of coastline that stretch along the South Atlantic and Indian Oceans; to the north by the neighbouring countri ...
, white men also used the ethnophaulism (lit. negro
In the English language, ''negro'' is a term historically used to denote persons considered to be of Black African heritage. The word ''negro'' means the color black in both Spanish and in Portuguese, where English took it from. The term can be ...
disease).[ Japan blamed ]sumo
is a form of competitive full-contact wrestling where a ''rikishi'' (wrestler) attempts to force his opponent out of a circular ring (''dohy┼Ź'') or into touching the ground with any body part other than the soles of his feet (usually by thr ...
wrestlers for bringing the disease home from a match in Taiwan
Taiwan, officially the Republic of China (ROC), is a country in East Asia, at the junction of the East and South China Seas in the northwestern Pacific Ocean, with the People's Republic of China (PRC) to the northwest, Japan to the nort ...
by calling it 'sumo flu' (), even though three top wrestlers died there.
World Health Organization
The World Health Organization (WHO) is a specialized agency of the United Nations responsible for international public health. The WHO Constitution states its main objective as "the attainment by all peoples of the highest possible level of h ...
'best practices' first published in 2015 now aim to prevent social stigma
Social stigma is the disapproval of, or discrimination against, an individual or group based on perceived characteristics that serve to distinguish them from other members of a society. Social stigmas are commonly related to culture, gender, rac ...
by no longer associating culturally significant names with new diseases, listing "Spanish flu" under "examples to be avoided".[ Many authors now eschew calling this the Spanish flu,][ instead using variations of '1918ŌĆō19/20 flu/influenza pandemic'.
]
Local names
Some language endonyms
An endonym (from Greek: , 'inner' + , 'name'; also known as autonym) is a common, ''native'' name for a geographical place, group of people, individual person, language or dialect, meaning that it is used inside that particular place, group, o ...
did not name specific regions or groups of people. Examples specific to this pandemic include: nd, 'Malibuzwe' (let enquiries be made concerning it), sw, 'Ugonjo huo kichwa na kukohoa na kiuno' (the disease of head and coughing and spine),
Other names
This outbreak was also commonly known as the 'great influenza epidemic', after the 'great war', a common name for World War I
World War I (28 July 1914 11 November 1918), often abbreviated as WWI, was one of the deadliest global conflicts in history. Belligerents included much of Europe, the Russian Empire, the United States, and the Ottoman Empire, with fightin ...
before World War II
World War II or the Second World War, often abbreviated as WWII or WW2, was a world war that lasted from 1939 to 1945. It involved the vast majority of the world's countriesŌĆöincluding all of the great powersŌĆöforming two opposin ...
. French military doctors originally called it 'disease 11' ().children's song
A children's song may be a nursery rhyme set to music, a song that children invent and share among themselves or a modern creation intended for entertainment, use in the home or education. Although children's songs have been recorded and studied ...
from the 1889ŌĆō90 flu pandemic was shortened and adapted into a skipping-rope rhyme
A skipping rhyme (occasionally skipping-rope rhyme or jump-rope rhyme), is a rhyme chanted by children while skipping. Such rhymes have been recorded in all cultures where skipping is played. Examples of English-language rhymes have been found ...
popular in 1918. It is a metaphor
A metaphor is a figure of speech that, for rhetorical effect, directly refers to one thing by mentioning another. It may provide (or obscure) clarity or identify hidden similarities between two different ideas. Metaphors are often compared wit ...
for the transmissibility of 'Influenza', where that name was clipped to the apheresis
Apheresis ( ß╝ĆŽå╬▒╬»Žü╬ĄŽā╬╣Žé (''aphairesis'', "a taking away")) is a medical technology in which the blood of a person is passed through an apparatus that separates out one particular constituent and returns the remainder to the circulation ...
'Enza':
History
Timeline
First wave of early 1918
The pandemic is conventionally marked as having begun on 4 March 1918 with the recording of the case of Albert Gitchell, an army cook at Camp Funston
Camp Funston is a U.S. Army training camp located on Fort Riley, southwest of Manhattan, Kansas. The camp was named for Brigadier General Frederick Funston (1865ŌĆō1917). It is one of sixteen such camps established at the outbreak of World War ...
in Kansas
Kansas () is a state in the Midwestern United States. Its capital is Topeka, and its largest city is Wichita. Kansas is a landlocked state bordered by Nebraska to the north; Missouri to the east; Oklahoma to the south; and Colorado to the ...
, United States, despite there having been cases before him. The disease had already been observed away in Haskell County as early as January 1918, prompting local doctor Loring Miner to warn the editors of the U.S. Public Health Service
The United States Public Health Service (USPHS or PHS) is a collection of agencies of the Department of Health and Human Services concerned with public health, containing nine out of the department's twelve operating divisions. The Assistant ...
's academic journal ''Public Health Reports''. Within days of the 4 March first case at Camp Funston, 522 men at the camp had reported sick. By 11 March 1918, the virus had reached Queens
Queens is a borough of New York City, coextensive with Queens County, in the U.S. state of New York. Located on Long Island, it is the largest New York City borough by area. It is bordered by the borough of Brooklyn at the western tip of Long ...
, New York. Failure to take preventive measures in March/April was later criticized.
As the U.S. had entered World War I
World War I (28 July 1914 11 November 1918), often abbreviated as WWI, was one of the deadliest global conflicts in history. Belligerents included much of Europe, the Russian Empire, the United States, and the Ottoman Empire, with fightin ...
, the disease quickly spread from Camp Funston, a major training ground for troops of the American Expeditionary Forces
The American Expeditionary Forces (A. E. F.) was a formation of the United States Army on the Western Front of World War I. The A. E. F. was established on July 5, 1917, in France under the command of General John J. Pershing. It fought alon ...
, to other U.S. Army
The United States Army (USA) is the land service branch of the United States Armed Forces. It is one of the eight U.S. uniformed services, and is designated as the Army of the United States in the U.S. Constitution.Article II, section 2, cl ...
camps and Europe, becoming an epidemic in the Midwest
The Midwestern United States, also referred to as the Midwest or the American Midwest, is one of four Census Bureau Region, census regions of the United States Census Bureau (also known as "Region 2"). It occupies the northern central part of ...
, East Coast
East Coast may refer to:
Entertainment
* East Coast hip hop, a subgenre of hip hop
* East Coast (ASAP Ferg song), "East Coast" (ASAP Ferg song), 2017
* East Coast (Saves the Day song), "East Coast" (Saves the Day song), 2004
* East Coast FM, a ra ...
, and French ports by April 1918, and reaching the Western Front by the middle of the month. It then quickly spread to the rest of France, Great Britain, Italy, and Spain and in May reached Wrocław
Wrocław (; german: Breslau, or . ; Silesian German: ''Brassel'') is a city in southwestern Poland and the largest city in the historical region of Silesia. It lies on the banks of the River Oder in the Silesian Lowlands of Central Europe, rou ...
and Odessa
Odesa (also spelled Odessa) is the third most populous city and municipality in Ukraine and a major seaport and transport hub located in the south-west of the country, on the northwestern shore of the Black Sea. The city is also the administrativ ...
. After the signing of the Treaty of Brest-Litovsk
The Treaty of Brest-Litovsk (also known as the Treaty of Brest in Russia) was a separate peace, separate peace treaty signed on 3 March 1918 between Russian SFSR, Russia and the Central Powers (German Empire, Germany, Austria-Hungary, Kingdom of ...
(March 1918), Germany started releasing Russian prisoners of war, who then brought the disease to their country. It reached North Africa, India, and Japan in May, and soon after had likely gone around the world as there had been recorded cases in Southeast Asia in April. In June an outbreak was reported in China. After reaching Australia in July, the wave started to recede.
The first wave of the flu lasted from the first quarter of 1918 and was relatively mild. Mortality rates were not appreciably above normal; in the United States ~75,000 flu-related deaths were reported in the first six months of 1918, compared to ~63,000 deaths during the same time period in 1915.World War I
World War I (28 July 1914 11 November 1918), often abbreviated as WWI, was one of the deadliest global conflicts in history. Belligerents included much of Europe, the Russian Empire, the United States, and the Ottoman Empire, with fightin ...
, with three-quarters of French troops, half the British forces, and over 900,000 German soldiers sick.
Deadly second wave of late 1918
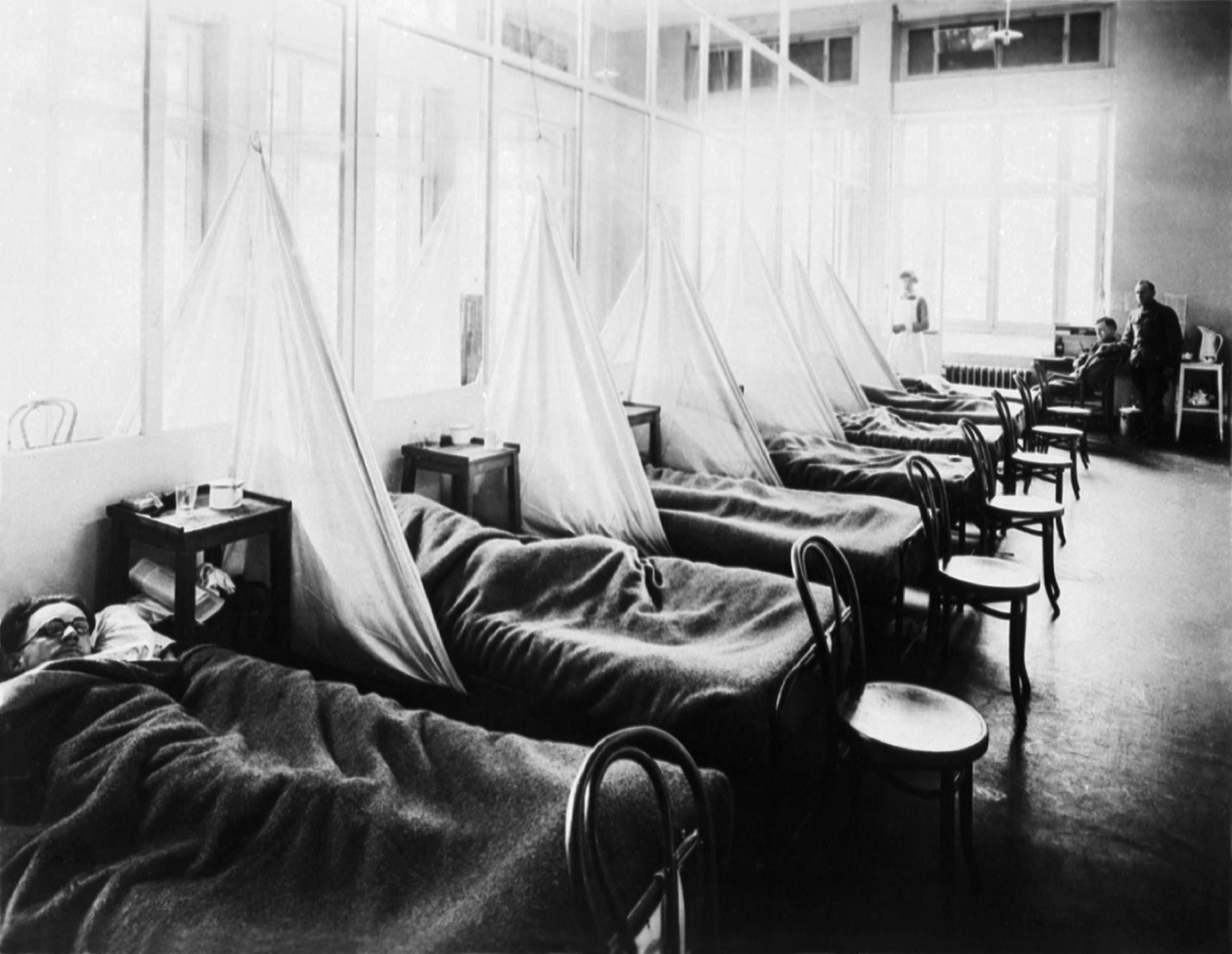
 The second wave began in the second half of August 1918, probably spreading to
The second wave began in the second half of August 1918, probably spreading to Boston
Boston (), officially the City of Boston, is the state capital and most populous city of the Commonwealth of Massachusetts, as well as the cultural and financial center of the New England region of the United States. It is the 24th- mo ...
and Freetown
Freetown is the capital and largest city of Sierra Leone. It is a major port city on the Atlantic Ocean and is located in the Western Area of the country. Freetown is Sierra Leone's major urban, economic, financial, cultural, educational and p ...
, Sierra Leone
Sierra Leone,)]. officially the Republic of Sierra Leone, is a country on the southwest coast of West Africa. It is bordered by Liberia to the southeast and Guinea surrounds the northern half of the nation. Covering a total area of , Sierra ...
, by ships from Brest, France, Brest, where it had likely arrived with American troops or French recruits for naval training. From the Boston Navy Yard
The Boston Navy Yard, originally called the Charlestown Navy Yard and later Boston Naval Shipyard, was one of the oldest shipbuilding facilities in the United States Navy. It was established in 1801 as part of the recent establishment of t ...
and Camp Devens (later renamed Fort Devens
Fort Devens is a United States Army Reserve military installation in the towns of Ayer and Shirley, in Middlesex County and Harvard in Worcester County in the U.S. state of Massachusetts. Due to extensive environmental contamination it was li ...
), about 30 miles west of Boston, other U.S. military sites were soon afflicted, as were troops being transported to Europe. Helped by troop movements, it spread over the next two months to all of North America, and then to Central and South America, also reaching Brazil and the Caribbean on ships. In July 1918, the Ottoman Empire
The Ottoman Empire, * ; is an archaic version. The definite article forms and were synonymous * and el, ą×╬ĖŽē╬╝╬▒╬Į╬╣╬║╬« ╬æŽģŽä╬┐╬║Žü╬▒Žä╬┐Žü╬»╬▒, Oth┼Źmanik─ō Avtokratoria, label=none * info page on book at Martin Luther University) ...
saw its first cases in some soldiers.South African Native Labour Corps
The South African Native Labour Corps (SANLC) was a force of workers formed in 1916 in response to a British request for workers at French ports. About 25,000 South Africans joined the Corps. The SANLC was utilized in various menial noncombat tas ...
returning from France. From there it spread around southern Africa and beyond the Zambezi
The Zambezi River (also spelled Zambeze and Zambesi) is the fourth-longest river in Africa, the longest east-flowing river in Africa and the largest flowing into the Indian Ocean from Africa. Its drainage basin covers , slightly less than hal ...
, reaching Ethiopia in November. On 15 September, New York City saw its first fatality from influenza. The Philadelphia Liberty Loans Parade
The Philadelphia Liberty Loans Parade was a parade in Philadelphia, Pennsylvania, on September 28, 1918, organized to promote government bonds that helped pay for the needs of Allied troops in World War I. More than 200,000 Philadelphians atten ...
, held in Philadelphia
Philadelphia, often called Philly, is the largest city in the Commonwealth of Pennsylvania, the sixth-largest city in the U.S., the second-largest city in both the Northeast megalopolis and Mid-Atlantic regions after New York City. Sinc ...
, Pennsylvania
Pennsylvania (; ( Pennsylvania Dutch: )), officially the Commonwealth of Pennsylvania, is a state spanning the Mid-Atlantic, Northeastern, Appalachian, and Great Lakes regions of the United States. It borders Delaware to its southeast, ...
, on 28 September 1918 to promote government bonds
A government bond or sovereign bond is a form of bond issued by a government to support public spending. It generally includes a commitment to pay periodic interest, called coupon payments'','' and to repay the face value on the maturity date ...
for World War I, resulted in 12,000 deaths after a major outbreak of the illness spread among people who had attended the parade.Arkhangelsk
Arkhangelsk (, ; rus, ąÉčĆčģą░╠üąĮą│ąĄą╗čīčüą║, p=╔Ér╦łxan╔Ī╩▓╔¬l╩▓sk), also known in English as Archangel and Archangelsk, is a types of inhabited localities in Russia, city and the administrative center of Arkhangelsk Oblast, Russia. It lies o ...
by the North Russia intervention
The North Russia intervention, also known as the Northern Russian expedition, the Archangel campaign, and the Murman deployment, was part of the Allied intervention in the Russian Civil War after the October Revolution. The intervention brought ...
, and then spread throughout Asia following the Russian Civil War
, date = October Revolution, 7 November 1917 ŌĆō Yakut revolt, 16 June 1923{{Efn, The main phase ended on 25 October 1922. Revolt against the Bolsheviks continued Basmachi movement, in Central Asia and Tungus Republic, the Far East th ...
and the Trans-Siberian railway
The Trans-Siberian Railway (TSR; , , ) connects European Russia to the Russian Far East. Spanning a length of over , it is the longest railway line in the world. It runs from the city of Moscow in the west to the city of Vladivostok in the ea ...
, reaching Iran (where it spread through the holy city of Mashhad
Mashhad ( fa, ┘ģž┤┘ćž», Ma┼Īhad ), also spelled Mashad, is the List of Iranian cities by population, second-most-populous city in Iran, located in the relatively remote north-east of the country about from Tehran. It serves as the capital of R ...
), and then later India in September, as well as China and Japan in October. The celebrations of the Armistice of 11 November 1918
The Armistice of 11 November 1918 was the armistice signed at Le Francport near Compi├©gne that ended fighting on land, sea, and air in World War I between the Entente and their last remaining opponent, Germany. Previous armistices ...
also caused outbreaks in Lima
Lima ( ; ), originally founded as Ciudad de Los Reyes (City of The Kings) is the capital and the largest city of Peru. It is located in the valleys of the Chill├│n River, Chill├│n, R├Łmac River, R├Łmac and Lur├Łn Rivers, in the desert zone of t ...
and Nairobi
Nairobi ( ) is the capital and largest city of Kenya. The name is derived from the Maasai phrase ''Enkare Nairobi'', which translates to "place of cool waters", a reference to the Nairobi River which flows through the city. The city proper ha ...
, but by December the wave was mostly over.
The second wave of the 1918 pandemic was much more deadly than the first. The first wave had resembled typical flu epidemics; those most at risk were the sick and elderly, while younger, healthier people recovered easily. October 1918 was the month with the highest fatality rate of the whole pandemic. In the United States, ~292,000 deaths were reported between SeptemberŌĆōDecember 1918, compared to ~26,000 during the same time period in 1915.Bombay
Mumbai (, ; also known as Bombay ŌĆö the official name until 1995) is the capital city of the Indian state of Maharashtra and the ''de facto'' financial centre of India. According to the United Nations, as of 2018, Mumbai is the second- ...
reported ~15,000 deaths in a population of 1.1 million.1918 flu pandemic in India
1918 flu pandemic in India was the outbreak of an unusually deadly influenza pandemic in British India between 1918 and 1920 as a part of the worldwide Spanish flu pandemic. Also referred to as the ''Bombay Influenza'' or the ''Bombay Fever'' in ...
was especially deadly, with an estimated 12.5ŌĆō20 million deaths in the last quarter of 1918 alone.
Third wave of 1919
In January 1919, the third wave of the flu hit Australia for the first time, where it killed around 12,000[
In the rest of the world, the third wave particularly affected Spain, Serbia, Mexico and Great Britain, resulting in hundreds of thousands of deaths.]Los Angeles
Los Angeles ( ; es, Los Ángeles, link=no , ), often referred to by its initials L.A., is the largest city in the state of California and the second most populous city in the United States after New York City, as well as one of the world' ...
,Memphis
Memphis most commonly refers to:
* Memphis, Egypt, a former capital of ancient Egypt
* Memphis, Tennessee, a major American city
Memphis may also refer to:
Places United States
* Memphis, Alabama
* Memphis, Florida
* Memphis, Indiana
* Memp ...
, Nashville
Nashville is the capital city of the U.S. state of Tennessee and the seat of Davidson County. With a population of 689,447 at the 2020 U.S. census, Nashville is the most populous city in the state, 21st most-populous city in the U.S., and the ...
, San Francisco
San Francisco (; Spanish language, Spanish for "Francis of Assisi, Saint Francis"), officially the City and County of San Francisco, is the commercial, financial, and cultural center of Northern California. The city proper is the List of Ca ...
, and St. Louis
St. Louis () is the second-largest city in Missouri, United States. It sits near the confluence of the Mississippi and the Missouri Rivers. In 2020, the city proper had a population of 301,578, while the bi-state metropolitan area, which e ...
.
Fourth wave of 1920
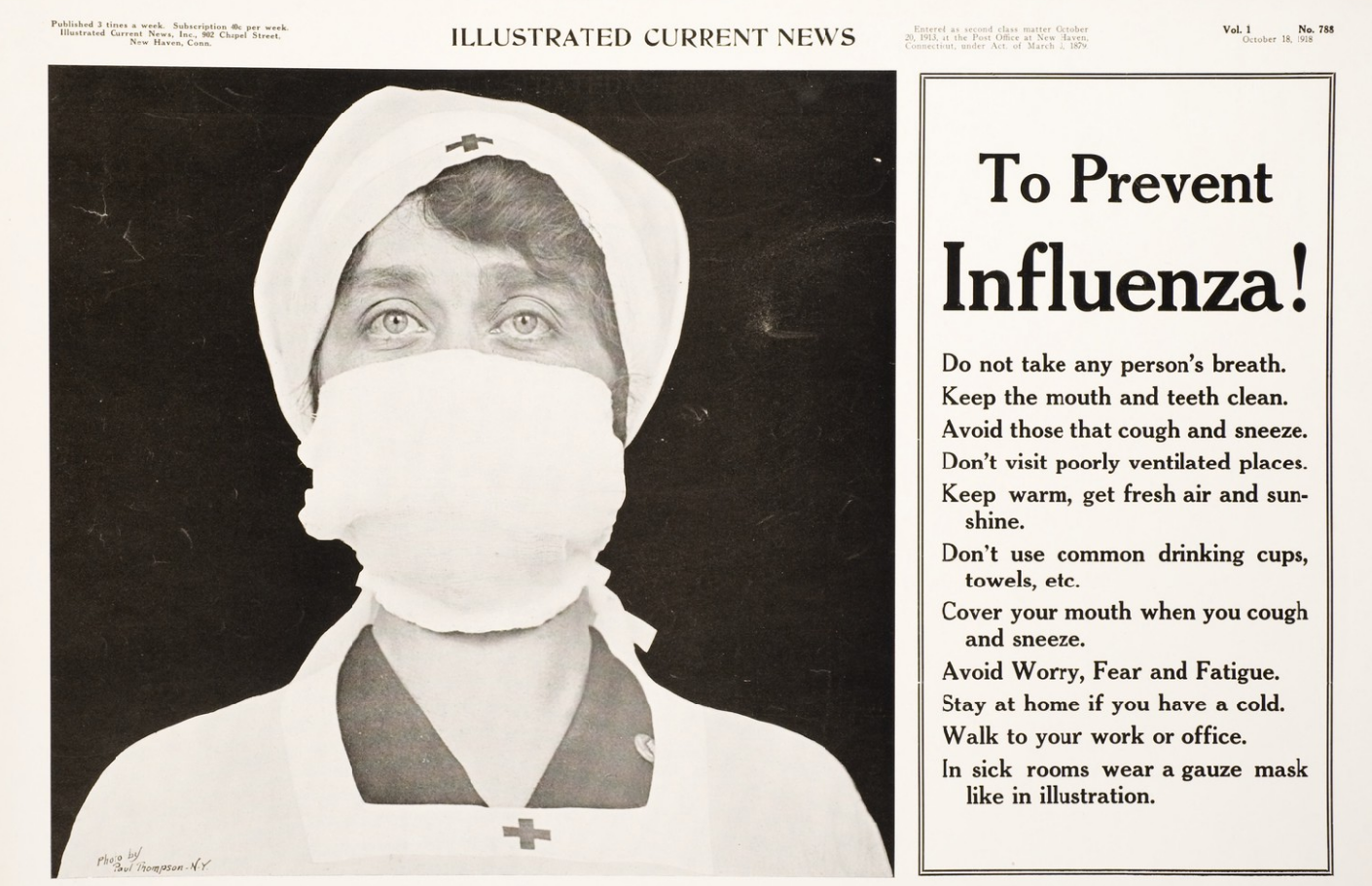 In the northern hemisphere, fears of a "recurrence" of the flu grew as fall approached. Experts cited the history of past flu epidemics, such as that of 1889ŌĆō1890, to predict that such a recurrence a year later was not unlikely, though not all agreed. In September,
In the northern hemisphere, fears of a "recurrence" of the flu grew as fall approached. Experts cited the history of past flu epidemics, such as that of 1889ŌĆō1890, to predict that such a recurrence a year later was not unlikely, though not all agreed. In September, U.S. Surgeon General
The surgeon general of the United States is the operational head of the United States Public Health Service Commissioned Corps (PHSCC) and thus the leading spokesperson on matters of public health in the federal government of the United States. Th ...
Rupert Blue
Rupert Lee Blue (May 30, 1868 ŌĆō April 12, 1948) was an American physician and soldier. He was the fourth Surgeon General of the United States from 1912 to 1920. He served as president of the American Medical Association in 1916–17.
Biog ...
said a return of the flu later in the year would "probably, but by no means certainly," occur. France had readied a public information campaign before the end of the summer, and Britain began preparations in the fall with the manufacture of vaccine.
In the United States, there were "almost continuously isolated or solitary cases" of flu throughout the spring and summer months.Senate
A senate is a deliberative assembly, often the upper house or chamber of a bicameral legislature. The name comes from the ancient Roman Senate (Latin: ''Senatus''), so-called as an assembly of the senior (Latin: ''senex'' meaning "the el ...
had sensed a turn in the tide (as the flu began to afflict their own families) and on 27 January passed an appropriations bill for $500,000 for the study of influenza.Carter Glass
Carter Glass (January 4, 1858 ŌĆō May 28, 1946) was an American newspaper publisher and Democratic politician from Lynchburg, Virginia. He represented Virginia in both houses of Congress and served as the United States Secretary of the Treasu ...
, Secretary of the Treasury
The United States secretary of the treasury is the head of the United States Department of the Treasury, and is the chief financial officer of the federal government of the United States. The secretary of the treasury serves as the principal a ...
, regarding the outbreaks occurring in large cities and the threat they posed to the rest of the country persuaded many senators to vote for the package. The fourth wave in the United States subsided as swiftly as it had appeared, reaching a peak in early February. "An epidemic of considerable proportions marked the early months of 1920", the U.S. Mortality Statistics would later note; according to data at this time, the epidemic resulted in one third as many deaths as the 1918ŌĆō1919 experience. New York City alone reported 6,374 deaths between December 1919 and April 1920, almost twice the number of the first wave in spring 1918.
The fourth wave in the United States subsided as swiftly as it had appeared, reaching a peak in early February. "An epidemic of considerable proportions marked the early months of 1920", the U.S. Mortality Statistics would later note; according to data at this time, the epidemic resulted in one third as many deaths as the 1918ŌĆō1919 experience. New York City alone reported 6,374 deaths between December 1919 and April 1920, almost twice the number of the first wave in spring 1918.Territory of Hawaii
The Territory of Hawaii or Hawaii Territory ( Hawaiian: ''Panalāʻau o Hawaiʻi'') was an organized incorporated territory of the United States that existed from April 30, 1900, until August 21, 1959, when most of its territory, excluding ...
experienced its peak of the pandemic in early 1920, recording 1,489 deaths from flu-related causes, compared with 615 in 1918 and 796 in 1919. Nenana, Alaska, had similarly avoided the extent of the pandemic for the previous two years, but by May 1920 an outbreak had consumed the town. Reports suggested that during the first two weeks of the month, the majority of the town's population became infected; 10% of the population were estimated to have died.
In winter and spring 1920, a fourth wave also occurred in various areas including Switzerland, Scandinavia,
End of the pandemic
By 1920, the virus that caused the pandemic evolved to become much less deadly and subsequently caused only ordinary seasonal flu. By 1921, deaths had returned to pre-pandemic levels.
Potential origins
Despite its name, historical and epidemiological
Epidemiology is the study and analysis of the distribution (who, when, and where), patterns and determinants of health and disease conditions in a defined population.
It is a cornerstone of public health, and shapes policy decisions and evidenc ...
data cannot identify the geographic origin of the Spanish flu. However, several theories have been proposed.
United States
The first confirmed cases originated in the United States. Historian Alfred W. Crosby stated in 2003 that the flu originated in Kansas
Kansas () is a state in the Midwestern United States. Its capital is Topeka, and its largest city is Wichita. Kansas is a landlocked state bordered by Nebraska to the north; Missouri to the east; Oklahoma to the south; and Colorado to the ...
, and author John M. Barry
John M. Barry (born 1947) is an American author and historian who has written books on the Great Mississippi Flood of 1927, the influenza pandemic of 1918, and the development of the modern form of the ideas of separation of church and state and ...
described a January 1918 outbreak in Haskell County, Kansas
Haskell County (county code HS) is a U.S. county, county located in the U.S. state of Kansas. As of the 2020 United States Census, 2020 census, the county population was 3,780. Its county seat and most populous city is Sublette, Kansas, Sublette ...
, as the point of origin in his 2004 article.
A 2018 study of tissue slides and medical reports led by evolutionary biology professor Michael Worobey found evidence against the disease originating from Kansas, as those cases were milder and had fewer deaths compared to the infections in New York City in the same period. The study did find evidence through phylogenetic analyses
In biology, phylogenetics (; from Greek ŽåŽģ╬╗╬«/ Žåß┐”╬╗╬┐╬Į [] "tribe, clan, race", and wikt:╬│╬Ą╬Į╬ĄŽä╬╣╬║ŽīŽé, ╬│╬Ą╬Į╬ĄŽä╬╣╬║ŽīŽé [] "origin, source, birth") is the study of the evolutionary history and relationships among or within groups o ...
that the virus likely had a North American origin, though it was not conclusive. In addition, the haemagglutinin glycoproteins of the virus suggest that it originated long before 1918, and other studies suggest that the reassortment
Reassortment is the mixing of the genetic material of a species into new combinations in different individuals. Several different processes contribute to reassortment, including assortment of chromosomes, and chromosomal crossover. It is particula ...
of the H1N1 virus likely occurred in or around 1915.
Europe
The major UK troop staging and hospital camp in ├ētaples
├ētaples or ├ētaples-sur-Mer (; vls, Stapel, lang; pcd, ├ētape) is a commune in the Pas-de-Calais department in northern France. It is a fishing and leisure port on the Canche river.
History
├ētaples takes its name from having been a medieval ...
in France has been theorized by virologist
Virology is the scientific study of biological viruses. It is a subfield of microbiology that focuses on their detection, structure, classification and evolution, their methods of infection and exploitation of host cells for reproduction, their ...
John Oxford as being at the center of the Spanish flu.Aldershot
Aldershot () is a town in Hampshire, England. It lies on heathland in the extreme northeast corner of the county, southwest of London. The area is administered by Rushmoor Borough Council. The town has a population of 37,131, while the Alders ...
,poison gas
Many gases have toxic properties, which are often assessed using the LC50 (median lethal dose) measure. In the United States, many of these gases have been assigned an NFPA 704 health rating of 4 (may be fatal) or 3 (may cause serious or perman ...
attacks, and other casualties of war, and 100,000 soldiers passed through the camp every day. It also was home to a piggery
Intensive pig farming, also known as pig factory farming, is the primary method of pig production, in which grower pigs are housed indoors in group-housing or straw-lined sheds, whilst pregnant sows are housed in gestation crates or pens and g ...
, and poultry
Poultry () are domesticated birds kept by humans for their eggs, their meat or their feathers. These birds are most typically members of the superorder Galloanserae (fowl), especially the order Galliformes (which includes chickens, quails, a ...
was regularly brought in from surrounding villages to feed the camp. Oxford and his team postulated that a precursor virus, harbored in birds, mutated
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, mitos ...
and then migrated to pigs kept near the front.Chinese Medical Association
In China, the practice of medicine is a mixture of government, charitable, and private institutions, while many people rely on traditional medicine. Until reforms in the late twentieth and early twenty-first century, physicians were quasi-governm ...
found evidence that the 1918 virus had been circulating in the European armies for months and possibly years before the 1918 pandemic.Austrian archives
The National Archives of Austria (german: Ûsterreichisches Staatsarchiv) Also known as the Austrian State Archives in Vienna is the central archive of the republic of Austria. On the basis of the Austrian Federal Archives Act, it stores the a ...
suggesting the influenza began in Austria in early 1917.
A 2009 study in ''Influenza and Other Respiratory Viruses
''Influenza and Other Respiratory Viruses'' is a peer-reviewed scientific journal covering virology, published by John Wiley & Sons for the International Society for Influenza and other Respiratory Virus Diseases. As of 2018, the editor is Benjami ...
'' found that Spanish flu mortality simultaneously peaked within the two-month period of October and November 1918 in all fourteen European countries analyzed, which is inconsistent with the pattern that researchers would expect if the virus had originated somewhere in Europe and then spread outwards.
China
In 1993, Claude Hannoun, the leading expert on the Spanish flu at the Pasteur Institute
The Pasteur Institute (french: Institut Pasteur) is a French non-profit private foundation dedicated to the study of biology, micro-organisms, diseases, and vaccines. It is named after Louis Pasteur, who invented pasteurization and vaccines f ...
, asserted the precursor virus was likely to have come from China
China, officially the People's Republic of China (PRC), is a country in East Asia. It is the world's most populous country, with a population exceeding 1.4 billion, slightly ahead of India. China spans the equivalent of five time zones and ...
and then mutated in the United States near Boston
Boston (), officially the City of Boston, is the state capital and most populous city of the Commonwealth of Massachusetts, as well as the cultural and financial center of the New England region of the United States. It is the 24th- mo ...
and from there spread to Brest, France, Brest, France
France (), officially the French Republic ( ), is a country primarily located in Western Europe. It also comprises of Overseas France, overseas regions and territories in the Americas and the Atlantic Ocean, Atlantic, Pacific Ocean, Pac ...
, Europe's battlefields, the rest of Europe, and the rest of the world, with Allied
An alliance is a relationship among people, groups, or states that have joined together for mutual benefit or to achieve some common purpose, whether or not explicit agreement has been worked out among them. Members of an alliance are called ...
soldiers and sailors as the main disseminators.Memorial University of Newfoundland
Memorial University of Newfoundland, also known as Memorial University or MUN (), is a public university in the province of Newfoundland and Labrador, based in St. John's, with satellite campuses in Corner Brook, elsewhere in Newfoundland and ...
in St. John's, based his conclusions on newly unearthed records. He found archival evidence that a respiratory illness that struck northern China (where the laborers came from) in November 1917 was identified a year later by Chinese health officials as identical to the Spanish flu.acquired immunity
The adaptive immune system, also known as the acquired immune system, is a subsystem of the immune system that is composed of specialized, systemic cells and processes that eliminate pathogens or prevent their growth. The acquired immune system ...
to the flu virus.Chinese Medical Association
In China, the practice of medicine is a mixture of government, charitable, and private institutions, while many people rely on traditional medicine. Until reforms in the late twentieth and early twenty-first century, physicians were quasi-governm ...
found no evidence that the 1918 virus was imported to Europe via Chinese and Southeast Asian soldiers and workers and instead found evidence of its circulation in Europe before the pandemic.
Epidemiology and pathology
Transmission and mutation
 The
The basic reproduction number
In epidemiology, the basic reproduction number, or basic reproductive number (sometimes called basic reproduction ratio or basic reproductive rate), denoted R_0 (pronounced ''R nought'' or ''R zero''), of an infection is the expected number of ...
of the virus was between 2 and 3. The close quarters and massive troop movements of World War I
World War I (28 July 1914 11 November 1918), often abbreviated as WWI, was one of the deadliest global conflicts in history. Belligerents included much of Europe, the Russian Empire, the United States, and the Ottoman Empire, with fightin ...
hastened the pandemic, and probably both increased transmission and augmented mutation. The war may also have reduced people's resistance to the virus. Some speculate the soldiers' immune systems were weakened by malnourishment, as well as the stresses of combat and chemical attacks, increasing their susceptibility. A large factor in the worldwide occurrence of the flu was increased travel. Modern transportation systems made it easier for soldiers, sailors, and civilian travelers to spread the disease. Another was lies and denial by governments, leaving the population ill-prepared to handle the outbreaks.natural selection
Natural selection is the differential survival and reproduction of individuals due to differences in phenotype. It is a key mechanism of evolution, the change in the heritable traits characteristic of a population over generations. Charle ...
favors a mild strain. Those who get very ill stay home, and those mildly ill continue with their lives, preferentially spreading the mild strain. In the trenches, natural selection was reversed. Soldiers with a mild strain stayed where they were, while the severely ill were sent on crowded trains to crowded field hospitals, spreading the deadlier virus. The second wave began, and the flu quickly spread around the world again. Consequently, during modern pandemics, health officials look for deadlier strains of a virus when it reaches places with social upheaval. The fact that most of those who recovered from first-wave infections had become immune
In biology, immunity is the capability of multicellular organisms to resist harmful microorganisms. Immunity involves both specific and nonspecific components. The nonspecific components act as barriers or eliminators of a wide range of pathogens ...
showed that it must have been the same strain of flu. This was most dramatically illustrated in Copenhagen
Copenhagen ( or .; da, K├Ėbenhavn ) is the capital and most populous city of Denmark, with a proper population of around 815.000 in the last quarter of 2022; and some 1.370,000 in the urban area; and the wider Copenhagen metropolitan ar ...
, which escaped with a combined mortality rate of just 0.29% (0.02% in the first wave and 0.27% in the second wave) because of exposure to the less-lethal first wave. For the rest of the population, the second wave was far more deadly; the most vulnerable people were those like the soldiers in the trenches ŌĆō adults who were young and fit.
After the lethal second wave struck in late 1918, new cases dropped abruptly. In Philadelphia, for example, 4,597 people died in the week ending 16 October, but by 11 November, influenza had almost disappeared from the city. One explanation for the rapid decline in the lethality of the disease is that doctors became more effective in the prevention and treatment of pneumonia that developed after the victims had contracted the virus. However, John Barry stated in his 2004 book '' The Great Influenza: The Epic Story of the Deadliest Plague In History'' that researchers have found no evidence to support this position. Another theory holds that the 1918 virus mutated extremely rapidly to a less lethal strain. Such evolution of influenza
Influenza, commonly known as "the flu", is an infectious disease caused by influenza viruses. Symptoms range from mild to severe and often include fever, runny nose, sore throat, muscle pain, headache, coughing, and fatigue. These symptoms ...
is a common occurrence: there is a tendency for pathogenic viruses to become less lethal with time, as the hosts of more dangerous strains tend to die out. Some fatal cases did continue into March 1919, killing one player in the 1919 Stanley Cup Finals.
Signs and symptoms
 The majority of the infected experienced only the typical flu symptoms of sore throat, headache, and fever, especially during the first wave. However, during the second wave, the disease was much more serious, often complicated by
The majority of the infected experienced only the typical flu symptoms of sore throat, headache, and fever, especially during the first wave. However, during the second wave, the disease was much more serious, often complicated by bacterial pneumonia
Bacterial pneumonia is a type of pneumonia caused by bacterial infection.
Types
Gram-positive
''Streptococcus pneumoniae'' () is the most common bacterial cause of pneumonia in all age groups except newborn infants. ''Streptococcus pneumoniae'' ...
, which was often the cause of death. This more serious type would cause ''heliotrope cyanosis'' to develop, whereby the skin would first develop two mahogany spots over the cheekbones which would then over a few hours spread to color the entire face blue, followed by black coloration first in the extremities and then further spreading to the limbs and the torso. After this, death would follow within hours or days due to the lungs being filled with fluids. Other signs and symptoms reported included spontaneous mouth and nosebleeds, miscarriages for pregnant women, a peculiar smell, teeth, and hair falling, delirium
Delirium (also known as acute confusional state) is an organically caused decline from a previous baseline of mental function that develops over a short period of time, typically hours to days. Delirium is a syndrome encompassing disturbances in ...
, dizziness, insomnia, loss of hearing or smell, and impaired vision. One observer wrote, "One of the most striking of the complications was hemorrhage
Bleeding, hemorrhage, haemorrhage or blood loss, is blood escaping from the circulatory system from damaged blood vessels. Bleeding can occur internally, or externally either through a natural opening such as the mouth, nose, ear, urethra, vag ...
from mucous membrane
A mucous membrane or mucosa is a membrane that lines various cavities in the body of an organism and covers the surface of internal organs. It consists of one or more layers of epithelial cells overlying a layer of loose connective tissue. It is ...
s, especially from the nose, stomach, and intestine. Bleeding from the ears and petechial hemorrhages in the skin also occurred". The severity of the symptoms was believed to be caused by cytokine storm
A cytokine storm, also called hypercytokinemia, is a physiological reaction in humans and other animals in which the innate immune system causes an uncontrolled and excessive release of pro-inflammatory signaling molecules called cytokines. Norm ...
s.
The majority of deaths were from bacterial pneumonia
Bacterial pneumonia is a type of pneumonia caused by bacterial infection.
Types
Gram-positive
''Streptococcus pneumoniae'' () is the most common bacterial cause of pneumonia in all age groups except newborn infants. ''Streptococcus pneumoniae'' ...
,secondary infection
An infection is the invasion of tissues by pathogens, their multiplication, and the reaction of host tissues to the infectious agent and the toxins they produce. An infectious disease, also known as a transmissible disease or communicable dise ...
associated with influenza. This pneumonia was itself caused by common upper respiratory-tract bacteria, which were able to get into the lungs via the damaged bronchial tubes
A bronchus is a passage or airway in the lower respiratory tract that conducts air into the lungs. The first or primary bronchi pronounced (BRAN-KAI) to branch from the trachea at the carina are the right main bronchus and the left main bronchus. ...
of the victims. The virus also killed people directly by causing massive hemorrhages and edema
Edema, also spelled oedema, and also known as fluid retention, dropsy, hydropsy and swelling, is the build-up of fluid in the body's Tissue (biology), tissue. Most commonly, the legs or arms are affected. Symptoms may include skin which feels t ...
in the lungs. Modern analysis has shown the virus to be particularly deadly because it triggers a cytokine storm (overreaction of the body's immune system). One group of researchers recovered the virus from the bodies of frozen victims and transfected
Transfection is the process of deliberately introducing naked or purified nucleic acids into eukaryotic cells. It may also refer to other methods and cell types, although other terms are often preferred: "transformation" is typically used to desc ...
animals with it. The animals suffered rapidly progressive respiratory failure
Respiratory failure results from inadequate gas exchange by the respiratory system, meaning that the arterial oxygen, carbon dioxide, or both cannot be kept at normal levels. A drop in the oxygen carried in the blood is known as hypoxemia; a rise ...
and death through a cytokine storm. The strong immune reactions of young adults were postulated to have ravaged the body, whereas the weaker immune reactions of children and middle-aged adults resulted in fewer deaths among those groups.
Misdiagnosis
Because the virus that caused the disease was too small to be seen under a microscope at the time, there were problems with correctly diagnosing it. The bacterium ''Haemophilus influenzae
''Haemophilus influenzae'' (formerly called Pfeiffer's bacillus or ''Bacillus influenzae'') is a Gram-negative, non-motile, coccobacillary, facultatively anaerobic, capnophilic pathogenic bacterium of the family Pasteurellaceae. The bacteria ...
'' was instead mistakenly thought to be the cause, as it was big enough to be seen and was present in many, though not all, patients. For this reason, a vaccine that was used against that bacillus did not make an infection rarer but did decrease the death rate.
During the deadly second wave there were also fears that it was in fact plague
Plague or The Plague may refer to:
Agriculture, fauna, and medicine
*Plague (disease), a disease caused by ''Yersinia pestis''
* An epidemic of infectious disease (medical or agricultural)
* A pandemic caused by such a disease
* A swarm of pes ...
, dengue fever
Dengue fever is a mosquito-borne tropical disease caused by the dengue virus. Symptoms typically begin three to fourteen days after infection. These may include a high fever, headache, vomiting, muscle and joint pains, and a characterist ...
, or cholera
Cholera is an infection of the small intestine by some strains of the bacterium ''Vibrio cholerae''. Symptoms may range from none, to mild, to severe. The classic symptom is large amounts of watery diarrhea that lasts a few days. Vomiting and ...
. Another common misdiagnosis was typhus
Typhus, also known as typhus fever, is a group of infectious diseases that include epidemic typhus, scrub typhus, and murine typhus. Common symptoms include fever, headache, and a rash. Typically these begin one to two weeks after exposure.
...
, which was common in circumstances of social upheaval, and was therefore also affecting Russia in the aftermath of the October Revolution
The October Revolution,. officially known as the Great October Socialist Revolution. in the Soviet Union, also known as the Bolshevik Revolution, was a revolution in Russia led by the Bolshevik Party of Vladimir Lenin that was a key moment ...
. In Chile
Chile, officially the Republic of Chile, is a country in the western part of South America. It is the southernmost country in the world, and the closest to Antarctica, occupying a long and narrow strip of land between the Andes to the east a ...
, the view of the country's elite was that the nation was in severe decline, and therefore doctors assumed that the disease was typhus caused by poor hygiene, and not an infectious one, causing a mismanaged response which did not ban mass gatherings.
The role of climate conditions
 Studies have shown that the immune system of Spanish flu victims was weakened by adverse climate conditions which were particularly unseasonably cold and wet for extended periods of time during the duration of the pandemic. This affected especially WWI troops exposed to incessant rains and lower-than-average temperatures for the duration of the conflict, and especially during the second wave of the pandemic. Ultra-high-resolution climate data combined with highly detailed mortality records analyzed at
Studies have shown that the immune system of Spanish flu victims was weakened by adverse climate conditions which were particularly unseasonably cold and wet for extended periods of time during the duration of the pandemic. This affected especially WWI troops exposed to incessant rains and lower-than-average temperatures for the duration of the conflict, and especially during the second wave of the pandemic. Ultra-high-resolution climate data combined with highly detailed mortality records analyzed at Harvard University
Harvard University is a private Ivy League research university in Cambridge, Massachusetts. Founded in 1636 as Harvard College and named for its first benefactor, the Puritan clergyman John Harvard, it is the oldest institution of higher le ...
and the Climate Change Institute at the University of Maine
The University of Maine (UMaine or UMO) is a Public university, public Land-grant university, land-grant research university in Orono, Maine. It was established in 1865 as the land-grant college of Maine and is the Flagship universities, flagshi ...
identified a severe climate anomaly that impacted Europe from 1914 to 1919, with several environmental indicators directly influencing the severity and spread of the Spanish flu pandemic.ANZAC
The Australian and New Zealand Army Corps (ANZAC) was a First World War army corps of the Mediterranean Expeditionary Force. It was formed in Egypt in December 1914, and operated during the Gallipoli campaign. General William Birdwood comma ...
troops suffered extremely cold temperatures despite the normally Mediterranean climate of the region. The climate anomaly likely influenced the migration of H1N1 avian vectors which contaminate bodies of water with their droppings, reaching 60% infection rates in autumn. The climate anomaly has been associated with an anthropogenic increase in atmospheric dust, due to the incessant bombardment; increased nucleation due to dust particles (cloud condensation nuclei
Cloud condensation nuclei (CCNs), also known as cloud seeds, are small particles typically 0.2 ┬Ąm, or one hundredth the size of a cloud droplet. CCNs are a unique subset of aerosols in the atmosphere on which water vapour condenses. This c ...
) contributed to increased precipitation.
Responses
Public health management
While systems for alerting public health authorities of infectious spread did exist in 1918, they did not generally include influenza, leading to a delayed response. Nevertheless, actions were taken. Maritime quarantines were declared on islands such as Iceland, Australia, and American Samoa, saving many lives. Social distancing
In public health, social distancing, also called physical distancing, (NB. Regula Venske is president of the PEN Centre Germany.) is a set of non-pharmaceutical interventions or measures intended to prevent the spread of a contagious disea ...
measures were introduced, for example closing schools, theatres, and places of worship, limiting public transportation, and banning mass gatherings. Wearing face masks became common in some places, such as Japan, though there were debates over their efficacy. There was also some resistance to their use, as exemplified by the Anti-Mask League of San Francisco
The Anti-Mask League was an organization formed in San Francisco, California to protest an ordinance which required people in that city to wear masks during the 1918 influenza pandemic. The ordinance it protested lasted less than one month before ...
. Vaccines were also developed, but as these were based on bacteria and not the actual virus, they could only help with secondary infections. The actual enforcement of various restrictions varied. To a large extent, the New York City health commissioner ordered businesses to open and close on staggered shifts to avoid overcrowding on the subways.
A later study found that measures such as banning mass gatherings and requiring the wearing of face masks could cut the death rate up to 50 percent, but this was dependent on their being imposed early in the outbreak and not being lifted prematurely.
Medical treatment
As there were no antiviral drug
Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Unlike most antibiotics, antiviral drugs do n ...
s to treat the virus, and no antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of ...
s to treat the secondary bacterial infections, doctors would rely on a random assortment of medicines with varying degrees of effectiveness, such as aspirin
Aspirin, also known as acetylsalicylic acid (ASA), is a nonsteroidal anti-inflammatory drug (NSAID) used to reduce pain, fever, and/or inflammation, and as an antithrombotic. Specific inflammatory conditions which aspirin is used to treat inc ...
, quinine
Quinine is a medication used to treat malaria and babesiosis. This includes the treatment of malaria due to ''Plasmodium falciparum'' that is resistant to chloroquine when artesunate is not available. While sometimes used for nocturnal leg cr ...
, arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, but ...
s, digitalis
''Digitalis'' ( or ) is a genus of about 20 species of herbaceous perennial plants, shrubs, and biennials, commonly called foxgloves.
''Digitalis'' is native to Europe, western Asia, and northwestern Africa. The flowers are tubular in sha ...
, strychnine
Strychnine (, , US chiefly ) is a highly toxic, colorless, bitter, crystalline alkaloid used as a pesticide, particularly for killing small vertebrates such as birds and rodents. Strychnine, when inhaled, swallowed, or absorbed through the eye ...
, epsom salts
Magnesium sulfate or magnesium sulphate (in English-speaking countries other than the US) is a chemical compound, a salt with the formula , consisting of magnesium cations (20.19% by mass) and sulfate anions . It is a white crystalline solid, s ...
, castor oil
Castor oil is a vegetable oil pressed from castor beans.
It is a colourless or pale yellow liquid with a distinct taste and odor. Its boiling point is and its density is 0.961 g/cm3. It includes a mixture of triglycerides in which about ...
, and iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ...
. Treatments of traditional medicine
Traditional medicine (also known as indigenous medicine or folk medicine) comprises medical aspects of traditional knowledge that developed over generations within the folk beliefs of various societies, including indigenous peoples, before the ...
, such as bloodletting
Bloodletting (or blood-letting) is the withdrawal of blood from a patient to prevent or cure illness and disease. Bloodletting, whether by a physician or by leeches, was based on an ancient system of medicine in which blood and other bodily flu ...
, ayurveda
Ayurveda () is an alternative medicine system with historical roots in the Indian subcontinent. The theory and practice of Ayurveda is pseudoscientific. Ayurveda is heavily practiced in India and Nepal, where around 80% of the population repo ...
, and kampo
, often known simply as , is the study of traditional Chinese medicine in Japan following its introduction, beginning in the 7th century. It was adapted and modified to suit Japanese culture and traditions. Traditional Japanese medicine use ...
were also applied.
Information dissemination
Due to World War I
World War I (28 July 1914 11 November 1918), often abbreviated as WWI, was one of the deadliest global conflicts in history. Belligerents included much of Europe, the Russian Empire, the United States, and the Ottoman Empire, with fightin ...
, many countries engaged in wartime censorship, and suppressed reporting of the pandemic. For example, the Italian newspaper ''Corriere della Sera
The ''Corriere della Sera'' (; en, "Evening Courier") is an Italian daily newspaper published in Milan with an average daily circulation of 410,242 copies in December 2015.
First published on 5 March 1876, ''Corriere della Sera'' is one of It ...
'' was prohibited from reporting daily death tolls. The newspapers of the time were also generally paternalistic
Paternalism is action that limits a person's or group's liberty or autonomy and is intended to promote their own good. Paternalism can also imply that the behavior is against or regardless of the will of a person, or also that the behavior expres ...
and worried about mass panic. Misinformation
Misinformation is incorrect or misleading information. It differs from disinformation, which is ''deliberately'' deceptive. Rumors are information not attributed to any particular source, and so are unreliable and often unverified, but can turn ou ...
also spread along with the disease. In Ireland there was a belief that noxious gases were rising from the mass graves of Flanders Fields
Flanders Fields is a common English name of the World War I battlefields in an area straddling the Belgian provinces of West Flanders and East Flanders as well as the French department of Nord-Pas-de-Calais, part of which makes up the area known as ...
and being "blown all over the world by winds". There were also rumors that the Germans were behind it, for example by poisoning the aspirin
Aspirin, also known as acetylsalicylic acid (ASA), is a nonsteroidal anti-inflammatory drug (NSAID) used to reduce pain, fever, and/or inflammation, and as an antithrombotic. Specific inflammatory conditions which aspirin is used to treat inc ...
manufactured by Bayer
Bayer AG (, commonly pronounced ; ) is a German multinational corporation, multinational pharmaceutical and biotechnology company and one of the largest pharmaceutical companies in the world. Headquartered in Leverkusen, Bayer's areas of busi ...
, or by releasing poison gas from U-boat
U-boats were naval submarines operated by Germany, particularly in the First and Second World Wars. Although at times they were efficient fleet weapons against enemy naval warships, they were most effectively used in an economic warfare role ...
s.
Mortality
Around the globe
 The Spanish flu infected around 500 million people, about one-third of the world's population. Estimates as to how many infected people died vary greatly, but the flu is regardless considered to be one of the deadliest pandemics in history. An early estimate from 1927 put global mortality at 21.6 million.
The Spanish flu infected around 500 million people, about one-third of the world's population. Estimates as to how many infected people died vary greatly, but the flu is regardless considered to be one of the deadliest pandemics in history. An early estimate from 1927 put global mortality at 21.6 million.American Journal of Epidemiology
The American Journal of Epidemiology (''AJE'') is a peer-reviewed journal for empirical research findings, opinion pieces, and methodological developments in the field of epidemiological research. The current editor-in-chief is Dr. Enrique Schist ...
'' estimated the total to be about 17 million,Influenza and Other Respiratory Viruses
''Influenza and Other Respiratory Viruses'' is a peer-reviewed scientific journal covering virology, published by John Wiley & Sons for the International Society for Influenza and other Respiratory Virus Diseases. As of 2018, the editor is Benjami ...
'' based on data from fourteen European countries estimated a total of 2.64 million excess deaths in Europe attributable to the Spanish flu during the major 1918ŌĆō1919 phase of the pandemic, in line with the three prior studies from 1991, 2002, and 2006 that calculated a European death toll of between 2 million and 2.3 million. This represents a mortality rate of about 1.1% of the European population ( 250 million in 1918), considerably higher than the mortality rate in the US, which the authors hypothesize is likely due to the severe effects of the war in Europe.India
India, officially the Republic of India (Hindi: ), is a country in South Asia. It is the seventh-largest country by area, the second-most populous country, and the most populous democracy in the world. Bounded by the Indian Ocean on the so ...
, about 5% of the population. The death toll in India's British-ruled districts was 13.88 million. Another estimate gives at least 12 million dead. The decade between 1911 and 1921 was the only census period in which India's population fell, mostly due to devastation of the Spanish flu pandemic. While India is generally described as the country most severely affected by the Spanish flu, at least one study argues that other factors may partially account for the very high excess mortality rates observed in 1918, citing unusually high 1917 mortality and wide regional variation (ranging from 0.47% to 6.66%).The Lancet
''The Lancet'' is a weekly peer-reviewed general medical journal and one of the oldest of its kind. It is also the world's highest-impact academic journal. It was founded in England in 1823.
The journal publishes original research articles, ...
'' also noted that Indian provinces had excess mortality rates ranging from 2.1% to 7.8%, stating: "Commentators at the time attributed this huge variation to differences in nutritional status and diurnal fluctuations in temperature."Finland
Finland ( fi, Suomi ; sv, Finland ), officially the Republic of Finland (; ), is a Nordic country in Northern Europe. It shares land borders with Sweden to the northwest, Norway to the north, and Russia to the east, with the Gulf of B ...
, 20,000 died out of 210,000 infected. In Sweden
Sweden, formally the Kingdom of Sweden,The United Nations Group of Experts on Geographical Names states that the country's formal name is the Kingdom of SwedenUNGEGN World Geographical Names, Sweden./ref> is a Nordic country located on ...
, 34,000 died.
In Japan
Japan ( ja, µŚźµ£¼, or , and formally , ''Nihonkoku'') is an island country in East Asia. It is situated in the northwest Pacific Ocean, and is bordered on the west by the Sea of Japan, while extending from the Sea of Okhotsk in the north ...
, 23 million people were affected, with at least 390,000 reported deaths. In the Dutch East Indies
The Dutch East Indies, also known as the Netherlands East Indies ( nl, Nederlands(ch)-Indië; ), was a Dutch colony consisting of what is now Indonesia. It was formed from the nationalised trading posts of the Dutch East India Company, which ...
(now Indonesia
Indonesia, officially the Republic of Indonesia, is a country in Southeast Asia and Oceania between the Indian and Pacific oceans. It consists of over 17,000 islands, including Sumatra, Java, Sulawesi, and parts of Borneo and New Guine ...
), 1.5 million were assumed to have died among 30 million inhabitants. In Tahiti
Tahiti (; Tahitian ; ; previously also known as Otaheite) is the largest island of the Windward group of the Society Islands in French Polynesia. It is located in the central part of the Pacific Ocean and the nearest major landmass is Austr ...
, 13% of the population died during one month. Similarly, in Western Samoa
Samoa, officially the Independent State of Samoa; sm, S─ümoa, and until 1997 known as Western Samoa, is a Polynesian island country consisting of two main islands ( Savai'i and Upolu); two smaller, inhabited islands ( Manono and Apolima); a ...
22% of the population of 38,000 died within two months.
In Istanbul
Istanbul ( , ; tr, ─░stanbul ), formerly known as Constantinople ( grc-gre, ╬ÜŽē╬ĮŽāŽä╬▒╬ĮŽä╬╣╬Į╬┐ŽŹŽĆ╬┐╬╗╬╣Žé; la, Constantinopolis), is the List of largest cities and towns in Turkey, largest city in Turkey, serving as the country's economic, ...
, capital of the Ottoman Empire, 6,403New Zealand
New Zealand ( mi, Aotearoa ) is an island country in the southwestern Pacific Ocean. It consists of two main landmassesŌĆöthe North Island () and the South Island ()ŌĆöand over 700 smaller islands. It is the sixth-largest island count ...
, the flu killed an estimated 6,400 Pakeha (or "New Zealanders primarily of European descent") and 2,500 indigenous Maori in six weeks, with M─üori dying at eight times the rate of Pakeha.
In the US, about 28% of the population of 105 million became infected, and 500,000 to 850,000 died (0.48 to 0.81 percent of the population). Native American tribes were particularly hard hit. In the Four Corners
The Four Corners is a region of the Southwestern United States consisting of the southwestern corner of Colorado, southeastern corner of Utah, northeastern corner of Arizona, and northwestern corner of New Mexico. The Four Corners area ...
area, there were 3,293 registered deaths among Native Americans. Entire Inuit
Inuit (; iu, ßÉāßōäßÉāßæ” 'the people', singular: Inuk, , dual: Inuuk, ) are a group of culturally similar indigenous peoples inhabiting the Arctic and subarctic regions of Greenland, Labrador, Quebec, Nunavut, the Northwest Territories ...
and Alaskan Native
Alaska Natives (also known as Alaskan Natives, Native Alaskans, Indigenous Alaskans, Aboriginal Alaskans or First Alaskans) are the indigenous peoples of Alaska and include I├▒upiat, Yupik, Aleut, Eyak, Tlingit, Haida, Tsimshian, and a numb ...
village communities died in Alaska
Alaska ( ; russian: ąÉą╗čÅčüą║ą░, Alyaska; ale, Alax╠ésxax╠é; ; ems, Alas'kaaq; Yup'ik: ''Alaskaq''; tli, An├Īaski) is a state located in the Western United States on the northwest extremity of North America. A semi-exclave of the U.S., ...
. In Canada, 50,000 died.
In Brazil
Brazil ( pt, Brasil; ), officially the Federative Republic of Brazil (Portuguese: ), is the largest country in both South America and Latin America. At and with over 217 million people, Brazil is the world's fifth-largest country by area ...
, 300,000 died, including president Rodrigues Alves
Francisco de Paula Rodrigues Alves, PC (; 7 July 1848 ŌĆō 16 January 1919) was a Brazilian politician who first served as president of the Province of S├Żo Paulo in 1887, then as Treasury minister in the 1890s. Rodrigues Alves was elected the ...
.
In Britain, as many as 250,000 died; in France, more than 400,000.
In Ghana
Ghana (; tw, Gaana, ee, Gana), officially the Republic of Ghana, is a country in West Africa. It abuts the Gulf of Guinea and the Atlantic Ocean to the south, sharing borders with Ivory Coast in the west, Burkina Faso in the north, and To ...
, the influenza epidemic killed at least 100,000 people. Tafari Makonnen (the future Haile Selassie
Haile Selassie I ( gez, ßēĆßŗ│ßłøßŗŖ ßŖĆßŗŁßłł ßłźßłŗßł┤, Q├żdamawi H├żyl├ż S╔Öllas├®, ; born Tafari Makonnen; 23 July 189227 August 1975) was Emperor of Ethiopia from 1930 to 1974. He rose to power as Regent Plenipotentiary of Ethiopia (' ...
, Emperor of Ethiopia
The emperor of Ethiopia ( gez, ßŖĢßīēßłĀ ßŖÉßīłßłźßēĄ, n╔Ögus├ż n├żg├żst, "King of Kings"), also known as the Atse ( am, ßŗÉߏä, "emperor"), was the hereditary monarchy, hereditary ruler of the Ethiopian Empire, from at least the 13th century ...
) was one of the first Ethiopians who contracted influenza but survived.Addis Ababa
Addis Ababa (; am, ßŖĀßŗ▓ßłĄ ßŖĀßēĀßēŻ, , new flower ; also known as , lit. "natural spring" in Oromo), is the capital and largest city of Ethiopia. It is also served as major administrative center of the Oromia Region. In the 2007 census, t ...
, range from 5,000 to 10,000, or higher.
The death toll in Russia
Russia (, , ), or the Russian Federation, is a List of transcontinental countries, transcontinental country spanning Eastern Europe and North Asia, Northern Asia. It is the List of countries and dependencies by area, largest country in the ...
has been estimated at 450,000, though the epidemiologists who suggested this number called it a "shot in the dark". If it is correct, Russia lost roughly 0.4% of its population, meaning it suffered the lowest influenza-related mortality in Europe
Europe is a large peninsula conventionally considered a continent in its own right because of its great physical size and the weight of its history and traditions. Europe is also considered a Continent#Subcontinents, subcontinent of Eurasia ...
. Another study considers this number unlikely, given that the country was in the grip of a civil war
A civil war or intrastate war is a war between organized groups within the same state (or country).
The aim of one side may be to take control of the country or a region, to achieve independence for a region, or to change government policies ...
, and the infrastructure of daily life had broken down; the study suggests that Russia's death toll was closer to 2%, or 2.7 million people.
Devastated communities
 Even in areas where mortality was low, so many adults were incapacitated that much of everyday life was hampered. Some communities closed all stores or required customers to leave orders outside. There were reports that healthcare workers could not tend the sick nor the gravediggers bury the dead because they too were ill. Mass graves were dug by
Even in areas where mortality was low, so many adults were incapacitated that much of everyday life was hampered. Some communities closed all stores or required customers to leave orders outside. There were reports that healthcare workers could not tend the sick nor the gravediggers bury the dead because they too were ill. Mass graves were dug by steam shovel
A steam shovel is a large steam-powered excavating machine designed for lifting and moving material such as rock and soil. It is the earliest type of power shovel or excavator. Steam shovels played a major role in public works in the 19th and e ...
and bodies buried without coffins in many places.
Bristol Bay
Bristol Bay ( esu, Iilgayaq, russian: ąŚą░ą╗ąĖą▓ ąæčĆąĖčüč鹊ą╗čīčüą║ąĖą╣) is the easternmost arm of the Bering Sea, at 57┬░ to 59┬░ North 157┬░ to 162┬░ West in Southwest Alaska. Bristol Bay is 400 km (250 mi) long and 290 km, ( ...
, a region of Alaska populated by indigenous people
Indigenous peoples are culturally distinct ethnic groups whose members are directly descended from the earliest known inhabitants of a particular geographic region and, to some extent, maintain the language and culture of those original people ...
, suffered a death rate of 40 percent of the total population, with some villages entirely disappearing.
Several Pacific island
Collectively called the Pacific Islands, the islands in the Pacific Ocean are further categorized into three major island groups: Melanesia, Micronesia, and Polynesia. Depending on the context, the term ''Pacific Islands'' may refer to one of se ...
territories were hit particularly hard. The pandemic reached them from New Zealand, which was too slow to implement measures to prevent ships, such as , carrying the flu from leaving its ports. From New Zealand, the flu reached Tonga
Tonga (, ; ), officially the Kingdom of Tonga ( to, Pule╩╗anga Fakatu╩╗i ╩╗o Tonga), is a Polynesian country and archipelago. The country has 171 islands ŌĆō of which 45 are inhabited. Its total surface area is about , scattered over in ...
(killing 8% of the population), Nauru
Nauru ( or ; na, Naoero), officially the Republic of Nauru ( na, Repubrikin Naoero) and formerly known as Pleasant Island, is an island country and microstate in Oceania, in the Central Pacific. Its nearest neighbour is Banaba Island in Ki ...
(16%), and Fiji
Fiji ( , ,; fj, Viti, ; Fiji Hindi: Óż½Óż╝Óż┐Óż£ÓźĆ, ''Fij─½''), officially the Republic of Fiji, is an island country in Melanesia, part of Oceania in the South Pacific Ocean. It lies about north-northeast of New Zealand. Fiji consists ...
(5%, 9,000 people). Worst affected was Western Samoa, formerly German Samoa
German Samoa (german: Deutsch-Samoa) was a German protectorate from 1900 to 1920, consisting of the islands of Upolu, Savai'i, Apolima and Manono, now wholly within the independent state of Samoa, formerly ''Western Samoa''. Samoa was the last ...
, which had been occupied by New Zealand in 1914. 90% of the population was infected; 30% of adult men, 22% of adult women, and 10% of children died. By contrast, Governor
A governor is an administrative leader and head of a polity or political region, ranking under the head of state and in some cases, such as governors-general, as the head of state's official representative. Depending on the type of political ...
John Martin Poyer
John Martin Poyer (1861 ŌĆō May 12, 1922) was the twelfth Naval Governor of American Samoa, from March 1, 1915 to June 10, 1919. He held the longest term of any American governor appointed over the territory by the United States Government. A N ...
prevented the flu from reaching neighboring American Samoa
American Samoa ( sm, Amerika S─ümoa, ; also ' or ') is an unincorporated territory of the United States located in the South Pacific Ocean, southeast of the island country of Samoa. Its location is centered on . It is east of the International ...
by imposing a blockade. The disease spread fastest through the higher social classes among the indigenous peoples, because of the custom of gathering oral tradition
Oral tradition, or oral lore, is a form of human communication wherein knowledge, art, ideas and cultural material is received, preserved, and transmitted orally from one generation to another. Vansina, Jan: ''Oral Tradition as History'' (1985 ...
from chiefs on their deathbeds; many community elders were infected through this process.
In Iran
Iran, officially the Islamic Republic of Iran, and also called Persia, is a country located in Western Asia. It is bordered by Iraq and Turkey to the west, by Azerbaijan and Armenia to the northwest, by the Caspian Sea and Turkmeni ...
, the mortality was very high: according to an estimate, between 902,400 and 2,431,000, or 8% to 22% of the total population died. The country was going through the Persian famine of 1917ŌĆō1919 concurrently.
In Ireland
Ireland ( ; ga, ├ēire ; Ulster Scots dialect, Ulster-Scots: ) is an island in the Atlantic Ocean, North Atlantic Ocean, in Northwestern Europe, north-western Europe. It is separated from Great Britain to its east by the North Channel (Grea ...
, during the worst 12 months, the Spanish flu accounted for one-third of all deaths.
In South Africa
South Africa, officially the Republic of South Africa (RSA), is the southernmost country in Africa. It is bounded to the south by of coastline that stretch along the South Atlantic and Indian Oceans; to the north by the neighbouring countri ...
it is estimated that about 300,000 people amounting to 6% of the population died within six weeks. Government actions in the early stages of the virus' arrival in the country in September 1918 are believed to have unintentionally accelerated its spread throughout the country. Almost a quarter of the working population of Kimberley, consisting of workers in the diamond mines, died. In British Somaliland
British Somaliland, officially the Somaliland Protectorate ( so, Dhulka Maxmiyada Soomaalida ee Biritishka), was a British Empire, British protectorate in present-day Somaliland. During its existence, the territory was bordered by Italian Soma ...
, one official estimated that 7% of the native population died. This huge death toll resulted from an extremely high infection rate
An infection rate (or incident rate) is the probability or risk of an infection in a population. It is used to measure the frequency of occurrence of new instances of infection within a population during a specific time period.
\text = K \time ...
of up to 50% and the extreme severity of the symptoms, suspected to be caused by cytokine storm
A cytokine storm, also called hypercytokinemia, is a physiological reaction in humans and other animals in which the innate immune system causes an uncontrolled and excessive release of pro-inflammatory signaling molecules called cytokines. Norm ...
s.
Less-affected areas
In the Pacific, American SamoaNew Caledonia
)
, anthem = ""
, image_map = New Caledonia on the globe (small islands magnified) (Polynesia centered).svg
, map_alt = Location of New Caledonia
, map_caption = Location of New Caledonia
, mapsize = 290px
, subdivision_type = Sovereign st ...
quarantine
A quarantine is a restriction on the movement of people, animals and goods which is intended to prevent the spread of disease or pests. It is often used in connection to disease and illness, preventing the movement of those who may have been ...
s. However, the outbreak was delayed into 1926 for American Samoa and 1921 for New Caledonia as the quarantine period ended.Maraj├│
Maraj├│ () is a large coastal island in the state of Par├Ī, Brazil. It is the main and largest of the islands in the Maraj├│ Archipelago. Maraj├│ Island is separated from the mainland by Maraj├│ Bay, Par├Ī River, smaller rivers (especially ...
, in Brazil's Amazon River Delta had not reported an outbreak. Saint Helena
Saint Helena () is a British overseas territory located in the South Atlantic Ocean. It is a remote volcanic tropical island west of the coast of south-western Africa, and east of Rio de Janeiro in South America. It is one of three constitu ...
also reported no deaths.
 Estimates for the death toll in China have varied widely,
Estimates for the death toll in China have varied widely,China
China, officially the People's Republic of China (PRC), is a country in East Asia. It is the world's most populous country, with a population exceeding 1.4 billion, slightly ahead of India. China spans the equivalent of five time zones and ...
may have experienced a relatively mild flu season in 1918 compared to other areas of the world.British
British may refer to:
Peoples, culture, and language
* British people, nationals or natives of the United Kingdom, British Overseas Territories, and Crown Dependencies.
** Britishness, the British identity and common culture
* British English, ...
-controlled Hong Kong
Hong Kong ( (US) or (UK); , ), officially the Hong Kong Special Administrative Region of the People's Republic of China ( abbr. Hong Kong SAR or HKSAR), is a city and special administrative region of China on the eastern Pearl River Delt ...
, Canton, Peking
}
Beijing ( ; ; ), alternatively romanized as Peking ( ), is the capital of the People's Republic of China. It is the center of power and development of the country. Beijing is the world's most populous national capital city, with over 21 ...
, Harbin
Harbin (; mnc, , v=Halbin; ) is a sub-provincial city and the provincial capital and the largest city of Heilongjiang province, People's Republic of China, as well as the second largest city by urban population after Shenyang and largest ...
and Shanghai
Shanghai (; , , Standard Mandarin pronunciation: ) is one of the four direct-administered municipalities of the People's Republic of China (PRC). The city is located on the southern estuary of the Yangtze River, with the Huangpu River flow ...
. These data were collected by the Chinese Maritime Customs Service
The Chinese Maritime Customs Service was a Chinese governmental tax collection agency and information service from its founding in 1854 until it split in 1949 into services operating in the Republic of China on Taiwan, and in the People's Republ ...
, which was largely staffed by non-Chinese foreigners, such as the British, French, and other European colonial
Colonial or The Colonial may refer to:
* Colonial, of, relating to, or characteristic of a colony or colony (biology)
Architecture
* American colonial architecture
* French Colonial
* Spanish Colonial architecture
Automobiles
* Colonial (1920 au ...
officials in China.Asia
Asia (, ) is one of the world's most notable geographical regions, which is either considered a continent in its own right or a subcontinent of Eurasia, which shares the continental landmass of Afro-Eurasia with Africa. Asia covers an area ...
.Asia
Asia (, ) is one of the world's most notable geographical regions, which is either considered a continent in its own right or a subcontinent of Eurasia, which shares the continental landmass of Afro-Eurasia with Africa. Asia covers an area ...
, such as Calcutta
Kolkata (, or , ; also known as Calcutta , List of renamed places in India#West Bengal, the official name until 2001) is the Capital city, capital of the Indian States and union territories of India, state of West Bengal, on the eastern ba ...
or Bombay
Mumbai (, ; also known as Bombay ŌĆö the official name until 1995) is the capital city of the Indian state of Maharashtra and the ''de facto'' financial centre of India. According to the United Nations, as of 2018, Mumbai is the second- ...
, where influenza was much more devastating.Shanghai
Shanghai (; , , Standard Mandarin pronunciation: ) is one of the four direct-administered municipalities of the People's Republic of China (PRC). The city is located on the southern estuary of the Yangtze River, with the Huangpu River flow ...
ŌĆō which had a population of over 2 million in 1918 ŌĆō there were only 266 recorded deaths from influenza
Influenza, commonly known as "the flu", is an infectious disease caused by influenza viruses. Symptoms range from mild to severe and often include fever, runny nose, sore throat, muscle pain, headache, coughing, and fatigue. These symptoms ...
among the Chinese population in 1918.Japan
Japan ( ja, µŚźµ£¼, or , and formally , ''Nihonkoku'') is an island country in East Asia. It is situated in the northwest Pacific Ocean, and is bordered on the west by the Sea of Japan, while extending from the Sea of Okhotsk in the north ...
and Taiwan
Taiwan, officially the Republic of China (ROC), is a country in East Asia, at the junction of the East and South China Seas in the northwestern Pacific Ocean, with the People's Republic of China (PRC) to the northwest, Japan to the nort ...
had reported a mortality rate from influenza around 0.45% and 0.69% respectively, higher than the mortality rate collected from data in Chinese port cities, such as Hong Kong (0.25%), Canton (0.32%), and Shanghai.
Patterns of fatality
 The pandemic mostly killed young adults. In 1918ŌĆō1919, 99% of pandemic influenza deaths in the U.S. occurred in people under 65, and nearly half of deaths were in young adults 20 to 40 years old. In 1920, the mortality rate among people under 65 had decreased sixfold to half the mortality rate of people over 65, but 92% of deaths still occurred in people under 65. This is unusual since influenza is typically most deadly to weak individuals, such as
The pandemic mostly killed young adults. In 1918ŌĆō1919, 99% of pandemic influenza deaths in the U.S. occurred in people under 65, and nearly half of deaths were in young adults 20 to 40 years old. In 1920, the mortality rate among people under 65 had decreased sixfold to half the mortality rate of people over 65, but 92% of deaths still occurred in people under 65. This is unusual since influenza is typically most deadly to weak individuals, such as infant
An infant or baby is the very young offspring of human beings. ''Infant'' (from the Latin word ''infans'', meaning 'unable to speak' or 'speechless') is a formal or specialised synonym for the common term ''baby''. The terms may also be used to ...
s under age two, adults over age 70, and the immunocompromise
Immunodeficiency, also known as immunocompromisation, is a state in which the immune system's ability to fight infectious diseases and cancer is compromised or entirely absent. Most cases are acquired ("secondary") due to extrinsic factors that a ...
d. In 1918, older adults may have had partial protection caused by exposure to the 1889ŌĆō1890 flu pandemic, known as the "Russian flu".[Hanssen, Olav. Unders├Ėkelser over influenzaens optr├”den specielt i Bergen 1918ŌĆō1922. Bg. 1923. 66 s. ill. (Haukeland sykehus. Med. avd. Arb. 2) (Klaus Hanssens fond. Skr. 3)] According to historian John M. Barry, the most vulnerable of all ŌĆō "those most likely, of the most likely", to die ŌĆō were pregnant women. He reported that in thirteen studies of hospitalized women in the pandemic, the death rate ranged from 23% to 71%. Of the pregnant women who survived childbirth, over one-quarter (26%) lost the child. Another oddity was that the outbreak was widespread in the summer and autumn (in the Northern Hemisphere
The Northern Hemisphere is the half of Earth that is north of the Equator. For other planets in the Solar System, north is defined as being in the same celestial hemisphere relative to the invariable plane of the solar system as Earth's Nort ...
); influenza is usually worse in winter.
There were also geographic patterns to the disease's fatality. Some parts of Asia had 30 times higher death rates than some parts of Europe, and generally, Africa and Asia had higher rates, while Europe and North America had lower ones. There was also great variation within continents, with three times higher mortality in Hungary and Spain compared to Denmark, two to three times higher chance of death in Sub-Saharan Africa compared to North Africa, and possibly up to ten times higher rates between the extremes of Asia. Cities were affected worse than rural areas. There were also differences between cities, which might have reflected exposure to the milder first wave giving immunity, as well as the introduction of social distancing measures.
Another major pattern was the differences between social classes. In Oslo
Oslo ( , , or ; sma, Oslove) is the capital and most populous city of Norway. It constitutes both a county and a municipality. The municipality of Oslo had a population of in 2022, while the city's greater urban area had a population of ...
, death rates were inversely correlated with apartment size, as the poorer people living in smaller apartments died at a higher rate. Social status was also reflected in the higher mortality among immigrant communities, with Italian Americans
Italian Americans ( it, italoamericani or ''italo-americani'', ) are Americans who have full or partial Italian ancestry. The largest concentrations of Italian Americans are in the urban Northeast and industrial Midwestern metropolitan areas, w ...
, a recently arrived group at the time, were nearly twice as likely to die compared to the average Americans. These disparities reflected worse diets, crowded living conditions, and problems accessing healthcare. Paradoxically, however, African Americans
African Americans (also referred to as Black Americans and Afro-Americans) are an ethnic group consisting of Americans with partial or total ancestry from sub-Saharan Africa. The term "African American" generally denotes descendants of ens ...
were relatively spared by the pandemic.
More men than women were killed by the flu, as they were more likely to go out and be exposed, while women would tend to stay at home. For the same reason men also were more likely to have pre-existing tuberculosis
Tuberculosis (TB) is an infectious disease usually caused by '' Mycobacterium tuberculosis'' (MTB) bacteria. Tuberculosis generally affects the lungs, but it can also affect other parts of the body. Most infections show no symptoms, in ...
, which severely worsened the chances of recovery. However, in India the opposite was true, potentially because Indian women were neglected with poorer nutrition, and were expected to care for the sick.
A study conducted by He ''et al''. (2011) used a mechanistic modeling approach to study the three waves of the 1918 influenza pandemic. They examined the factors that underlie variability in temporal patterns and their correlation to patterns of mortality and morbidity. Their analysis suggests that temporal variations in transmission rate provide the best explanation, and the variation in transmission required to generate these three waves is within biologically plausible values. Another study by He ''et al''. (2013) used a simple epidemic model
Compartmental models are a very general modelling technique. They are often applied to the mathematical modelling of infectious diseases. The population is assigned to compartments with labels ŌĆō for example, S, I, or R, (Susceptible, Infectious, ...
incorporating three factors to infer the cause of the three waves of the 1918 influenza pandemic. These factors were school opening and closing, temperature changes throughout the outbreak, and human behavioral changes in response to the outbreak. Their modeling results showed that all three factors are important, but human behavioral responses showed the most significant effects.
Effects
World War I
Academic Andrew Price-Smith has made the argument that the virus helped tip the balance of power in the latter days of the war towards the Allied cause. He provides data that the viral waves hit the Central Powers
The Central Powers, also known as the Central Empires,german: Mittelm├żchte; hu, K├Čzponti hatalmak; tr, ─░ttifak Devletleri / ; bg, ą”ąĄąĮčéčĆą░ą╗ąĮąĖ čüąĖą╗ąĖ, translit=Tsentralni sili was one of the two main coalitions that fought in ...
before the Allied powers and that both morbidity
A disease is a particular abnormal condition that negatively affects the structure or function of all or part of an organism, and that is not immediately due to any external injury. Diseases are often known to be medical conditions that a ...
and mortality in Weimar Republic, Germany and First Austrian Republic, Austria were considerably higher than in Britain and France
France (), officially the French Republic ( ), is a country primarily located in Western Europe. It also comprises of Overseas France, overseas regions and territories in the Americas and the Atlantic Ocean, Atlantic, Pacific Ocean, Pac ...
. A 2006 ''Lancet'' study corroborates higher excess mortality rates in Germany (0.76%) and Austria (1.61%) compared to Britain (0.34%) and France (0.75%).
Economic
 Many businesses in the entertainment and service industries suffered losses in revenue, while the healthcare industry reported profit gains. Historian Nancy Bristow has argued that the pandemic, when combined with the increasing number of women attending college, contributed to the success of women in the field of nursing. This was due in part to the failure of medical doctors, who were predominantly men, to contain and prevent the illness. Nursing staff, who were mainly women, celebrated the success of their patient care and did not associate the spread of the disease with their work.
A 2020 study found that US cities that implemented early and extensive non-medical measures (quarantine, etc.) suffered no additional adverse economic effects due to implementing those measures,
however, the validity of this study has been questioned because of the coincidence of WWI and other problems with data reliability.
Many businesses in the entertainment and service industries suffered losses in revenue, while the healthcare industry reported profit gains. Historian Nancy Bristow has argued that the pandemic, when combined with the increasing number of women attending college, contributed to the success of women in the field of nursing. This was due in part to the failure of medical doctors, who were predominantly men, to contain and prevent the illness. Nursing staff, who were mainly women, celebrated the success of their patient care and did not associate the spread of the disease with their work.
A 2020 study found that US cities that implemented early and extensive non-medical measures (quarantine, etc.) suffered no additional adverse economic effects due to implementing those measures,
however, the validity of this study has been questioned because of the coincidence of WWI and other problems with data reliability.
Long-term effects
A 2006 study in the ''Journal of Political Economy'' found that "cohorts ''in utero'' during the pandemic displayed reduced educational attainment, increased rates of physical disability, lower income, lower socioeconomic status, and higher transfer payments received compared with other birth cohorts." A 2018 study found that the pandemic reduced educational attainment in populations. The flu has also been linked to the outbreak of encephalitis lethargica in the 1920s.
Survivors faced an elevated mortality risk. Some survivors did not fully recover from physiological conditions resulting from infection.
Legacy
 Despite the high morbidity and mortality rates that resulted from the epidemic, the Spanish flu began to fade from public awareness over the decades until the arrival of news about Avian influenza, bird flu and other pandemics in the 1990s and 2000s. This has led some historians to label the Spanish flu a "forgotten pandemic". However, this label has been challenged by the historian Guy Beiner, who has charted a complex history of social and cultural forgetting, demonstrating how the pandemic was overshadowed by the commemoration of the First World War and mostly neglected in mainstream historiography, yet was remembered in private and local traditions across the globe.
There are various theories of why the Spanish flu was "forgotten". The rapid pace of the pandemic, which killed most of its victims in the United States within less than nine months, resulted in limited media coverage. The general population was familiar with patterns of pandemic disease in the late 19th and early 20th centuries: typhoid, yellow fever, diphtheria, and cholera all occurred near the same time. These outbreaks probably lessened the significance of the influenza pandemic for the public. In some areas, the flu was not reported on, the only mention being that of advertisements for medicines claiming to cure it.
Additionally, the outbreak coincided with the deaths and media focus on the First World War. Another explanation involves the age group affected by the disease. The majority of fatalities, from both the war and the epidemic, were among young adults. The high number of war-related deaths of young adults may have overshadowed the deaths caused by flu.
When people read the obituaries, they saw the war or postwar deaths and the deaths from the influenza side by side. Particularly in Europe, where the war's toll was high, the flu may not have had a tremendous psychological impact or may have seemed an extension of the war's tragedies. The duration of the pandemic and the war could have also played a role. The disease would usually only affect a particular area for a month before leaving. The war, however, had initially been expected to end quickly but lasted for four years by the time the pandemic struck.
Despite the high morbidity and mortality rates that resulted from the epidemic, the Spanish flu began to fade from public awareness over the decades until the arrival of news about Avian influenza, bird flu and other pandemics in the 1990s and 2000s. This has led some historians to label the Spanish flu a "forgotten pandemic". However, this label has been challenged by the historian Guy Beiner, who has charted a complex history of social and cultural forgetting, demonstrating how the pandemic was overshadowed by the commemoration of the First World War and mostly neglected in mainstream historiography, yet was remembered in private and local traditions across the globe.
There are various theories of why the Spanish flu was "forgotten". The rapid pace of the pandemic, which killed most of its victims in the United States within less than nine months, resulted in limited media coverage. The general population was familiar with patterns of pandemic disease in the late 19th and early 20th centuries: typhoid, yellow fever, diphtheria, and cholera all occurred near the same time. These outbreaks probably lessened the significance of the influenza pandemic for the public. In some areas, the flu was not reported on, the only mention being that of advertisements for medicines claiming to cure it.
Additionally, the outbreak coincided with the deaths and media focus on the First World War. Another explanation involves the age group affected by the disease. The majority of fatalities, from both the war and the epidemic, were among young adults. The high number of war-related deaths of young adults may have overshadowed the deaths caused by flu.
When people read the obituaries, they saw the war or postwar deaths and the deaths from the influenza side by side. Particularly in Europe, where the war's toll was high, the flu may not have had a tremendous psychological impact or may have seemed an extension of the war's tragedies. The duration of the pandemic and the war could have also played a role. The disease would usually only affect a particular area for a month before leaving. The war, however, had initially been expected to end quickly but lasted for four years by the time the pandemic struck.
In fiction and other literature
The Spanish flu has been represented in numerous works of fiction:
* Katherine Anne Porter's novella ''Pale Horse, Pale Rider'', published under the same title in a 1939 collection of three works
* 1918 (1985 film), ''1918'', a 1985 American drama film.
* ''The Last Town on Earth'', a 2006 novel.
* ''Spanish Flu: The Forgotten Fallen'', a 2009 British television series.
* ''Downton Abbey'', a 2010 British historical drama television series.
* ''Vampyr (video game), Vampyr'', a 2018 video game.
* ''The Pull of the Stars'', a 2020 novel by Emma Donoghue set in Dublin during the Spanish flu
* ''Resident Evil Village'', a 2021 video game.
* In ''Frankie Drake Mysteries'', series 4, episode 9, the plot revolves around an apparent outbreak of Spanish flu in a theatre where there is going to be a performance which some think risqu├®.
Mary McCarthy (author), Mary McCarthy referred to it in her memoir ''Memories of a Catholic Girlhood'' (1957), as she and her three brothers were orphaned by their parents' deaths from the Spanish flu.
Comparison with other pandemics
The Spanish flu killed a much lower percentage of the world's population than the Black Death, which lasted for many more years.
Research
 The origin of the Spanish flu pandemic, and the relationship between the near-simultaneous outbreaks in humans and swine, have been controversial. One hypothesis is that the virus strain originated at Fort Riley, Kansas, in viruses in poultry and swine which the fort bred for food; the soldiers were then sent from Fort Riley around the world, where they spread the disease. Similarities between a reconstruction of the virus and avian viruses, combined with the human pandemic preceding the first reports of influenza in swine, led researchers to conclude the influenza virus jumped directly from birds to humans, and swine caught the disease from humans.
The origin of the Spanish flu pandemic, and the relationship between the near-simultaneous outbreaks in humans and swine, have been controversial. One hypothesis is that the virus strain originated at Fort Riley, Kansas, in viruses in poultry and swine which the fort bred for food; the soldiers were then sent from Fort Riley around the world, where they spread the disease. Similarities between a reconstruction of the virus and avian viruses, combined with the human pandemic preceding the first reports of influenza in swine, led researchers to conclude the influenza virus jumped directly from birds to humans, and swine caught the disease from humans. An effort to recreate the Spanish flu strain (a subtype of avian strain H1N1) was a collaboration among the Armed Forces Institute of Pathology, the USDA Agricultural Research Service, ARS Southeast Poultry Research Laboratory, and Mount Sinai School of Medicine in New York City. The effort resulted in the announcement (on 5 October 2005) that the group had successfully determined the virus's genetic sequence, using historic tissue samples recovered by pathologist Johan Hultin from an Inuit female flu victim buried in the Alaskan permafrost and samples preserved from American soldiers Roscoe Vaughan and James Downs.
On 18 January 2007, Kobasa et al. (2007) reported that monkeys (''Macaca fascicularis'') infected with the recreated flu strain exhibited classic symptoms of the 1918 pandemic, and died from cytokine storms ŌĆō an overreaction of the immune system. This may explain why the Spanish flu had its surprising effect on younger, healthier people, as a person with a stronger immune system would potentially have a stronger overreaction.
On 16 September 2008, the body of British politician and diplomat Mark Sykes, Sir Mark Sykes was exhumed to study the RNA of the flu virus in efforts to understand the genetic structure of modern H5N1 bird flu. Sykes had been buried in 1919 in a lead coffin which scientists hoped had helped preserve the virus. The coffin was found to be split and the cadaver badly decomposed; nonetheless, samples of lung and brain tissue were taken.
In December 2008, research by Yoshihiro Kawaoka of the University of Wisconsin linked the presence of three specific genes (termed PA, PB1, and PB2) and a nucleoprotein derived from Spanish flu samples to the ability of the flu virus to invade the lungs and cause pneumonia. The combination triggered similar symptoms in animal testing.
In June 2010, a team at the Mount Sinai School of Medicine reported the 2009 flu pandemic vaccine provided some cross-protection against the Spanish flu pandemic strain.
One of the few things known for certain about influenza in 1918 and for some years after was that it was, except in the laboratory, exclusively a disease of human beings.
In 2013, the AIR Worldwide Research and Modeling Group "characterized the historic 1918 pandemic and estimated the effects of a similar pandemic occurring today using the AIR Pandemic Flu Model". In the model, "a modern-day 'Spanish flu' event would result in additional life insurance losses of between US$15.3ŌĆō27.8 billion in the United States alone", with 188,000ŌĆō337,000 deaths in the United States.
In 2018, Michael Worobey, an evolutionary biology professor at the University of Arizona who is examining the history of the 1918 pandemic, revealed that he obtained tissue slides created by William Rolland, a physician who reported on a respiratory illness likely to be the virus while a pathologist in the British military during World War One. Rolland had authored an article in the ''Lancet (journal), Lancet'' during 1917 about a respiratory illness outbreak beginning in 1916 in ├ētaples, France. Worobey traced recent references to that article to family members who had retained slides that Rolland had prepared during that time. Worobey extracted tissue from the slides to potentially reveal more about the origin of the pathogen.
An effort to recreate the Spanish flu strain (a subtype of avian strain H1N1) was a collaboration among the Armed Forces Institute of Pathology, the USDA Agricultural Research Service, ARS Southeast Poultry Research Laboratory, and Mount Sinai School of Medicine in New York City. The effort resulted in the announcement (on 5 October 2005) that the group had successfully determined the virus's genetic sequence, using historic tissue samples recovered by pathologist Johan Hultin from an Inuit female flu victim buried in the Alaskan permafrost and samples preserved from American soldiers Roscoe Vaughan and James Downs.
On 18 January 2007, Kobasa et al. (2007) reported that monkeys (''Macaca fascicularis'') infected with the recreated flu strain exhibited classic symptoms of the 1918 pandemic, and died from cytokine storms ŌĆō an overreaction of the immune system. This may explain why the Spanish flu had its surprising effect on younger, healthier people, as a person with a stronger immune system would potentially have a stronger overreaction.
On 16 September 2008, the body of British politician and diplomat Mark Sykes, Sir Mark Sykes was exhumed to study the RNA of the flu virus in efforts to understand the genetic structure of modern H5N1 bird flu. Sykes had been buried in 1919 in a lead coffin which scientists hoped had helped preserve the virus. The coffin was found to be split and the cadaver badly decomposed; nonetheless, samples of lung and brain tissue were taken.
In December 2008, research by Yoshihiro Kawaoka of the University of Wisconsin linked the presence of three specific genes (termed PA, PB1, and PB2) and a nucleoprotein derived from Spanish flu samples to the ability of the flu virus to invade the lungs and cause pneumonia. The combination triggered similar symptoms in animal testing.
In June 2010, a team at the Mount Sinai School of Medicine reported the 2009 flu pandemic vaccine provided some cross-protection against the Spanish flu pandemic strain.
One of the few things known for certain about influenza in 1918 and for some years after was that it was, except in the laboratory, exclusively a disease of human beings.
In 2013, the AIR Worldwide Research and Modeling Group "characterized the historic 1918 pandemic and estimated the effects of a similar pandemic occurring today using the AIR Pandemic Flu Model". In the model, "a modern-day 'Spanish flu' event would result in additional life insurance losses of between US$15.3ŌĆō27.8 billion in the United States alone", with 188,000ŌĆō337,000 deaths in the United States.
In 2018, Michael Worobey, an evolutionary biology professor at the University of Arizona who is examining the history of the 1918 pandemic, revealed that he obtained tissue slides created by William Rolland, a physician who reported on a respiratory illness likely to be the virus while a pathologist in the British military during World War One. Rolland had authored an article in the ''Lancet (journal), Lancet'' during 1917 about a respiratory illness outbreak beginning in 1916 in ├ētaples, France. Worobey traced recent references to that article to family members who had retained slides that Rolland had prepared during that time. Worobey extracted tissue from the slides to potentially reveal more about the origin of the pathogen.
Sex differences in mortality
The high mortality rate of the influenza pandemic is one aspect that sets the pandemic apart from other disease outbreaks. Another factor is the higher mortality rate of men compared with women. Men with an underlying condition were at significantly more risk. Tuberculosis was one of the deadliest diseases in the 1900s, and killed more men than women. But with the spread of influenza disease, the cases of tuberculosis cases in men decreased. Many scholars have noted that tuberculosis increased the mortality rate of influenza in males, decreasing their life expectancy. During the 1900s tuberculosis was more common in males than females, but studies show that when influenza spread the tuberculosis mortality rate among females changed. The death rate of tuberculosis in females increased significantly and would continue to decline until post-pandemic.
Death rates were particularly high in those aged 20ŌĆō35. The only comparable disease to this was the Black Death, black death, bubonic plague in the 1300s. As other studies have shown, tuberculosis and influenza had Comorbidity, comorbidities and one affected the other. The ages of males dying of the flu show that tuberculosis was a factor, and as males primarily had this disease at the time of the pandemic, they had a higher mortality rate. Life expectancy dropped in males during the pandemic but then increased two years after the pandemic.
Island of Newfoundland
One major cause of the spread of influenza was social behavior. Men had more social variation and were mobile more than women due to their work. Even though there was a higher mortality rate in males, each region showed different results, due to such factors as Malnutrition, nutritional deficiency. In Newfoundland and Labrador, Newfoundland, the pandemic spread was highly variable. Influenza did not discriminate who was infected, indeed it attacked the Socioeconomic status, socioeconomic status of people. Although social variability allowed the disease to move quickly geographically, it tended to spread faster and affect men more than women due to labor and social contact. Newfoundland's leading cause of death before the pandemic was tuberculosis and this is known to be a severe underlying condition for people and increases the , mortality rate when infected by the influenza disease. There was diverse labor in Newfoundland, men and women had various occupations that involved day-to-day interaction. But, fishing had a major role in the economy and so males were more mobile than females and had more contact with other parts of the world. The spread of the pandemic is known to have begun in the spring of 1918, but Newfoundland didn't see the deadly wave until June or July, which aligns with the high demand for employment in the fishery. The majority of men were working along the coast during the summer and it was typical for entire families to move to Newfoundland and work. Studies show a much higher mortality rate in males compared with females. But, during the first, second, and third waves of the pandemic, the mortality shifted. During the first wave, men had a higher mortality rate, but the mortality rate of females increased and was higher during the second and third waves. The female population was larger in certain regions of Newfoundland and therefore had a bigger impact on the death rate.
Influenza pandemic among Canadian soldiers
Records indicate the most deaths during the first wave of the pandemic were among young men in their 20s, which reflects the age of enlistment in the war. The mobility of young men during 1918 was linked to the spread of influenza and the biggest wave of the epidemic. In late 1917 and throughout 1918, thousands of male troops gathered at the Halifax port before heading to Europe. Any soldier that was ill and could not depart was added to the population of Halifax, Nova Scotia, Halifax, which increased the case rate of influenza among men during the war. To determine the cause of the death during the pandemic, war scientists used the Commonwealth War Graves Commission (CWGC), which reported under 2 million men and women died during the wars, with a record of those who died from 1917 to 1918. The movement of soldiers during this time and the transportation from United States between Canada likely had a significant effect on the spread of the pandemic.
See also
*
*
*
*
*
* List of epidemics
* List of Spanish flu cases
Footnotes
References
Citations
Bibliography
*
* ŌĆō Open access material by the Psychiatry and Behavioral Sciences at Health Sciences Research Commons.
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
summary
by Infectious Diseases Society of America and ScienceDaily, 3 October 2009)
*
*
*
*
*
*
Further reading
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
; Videos
We Heard the Bells: The Influenza of 1918
ŌĆō NIH interviews with Spanish Flu survivors.
Presentation by Nancy Bristow on the Influenza Pandemic and World War I, November 1, 2019
C-SPAN
*
External links
*
The American Influenza Epidemic of 1918ŌĆō1919: A Digital Encyclopedia
Largest digital collection of newspapers, archival manuscripts, and interpretive essays exploring the impact of the epidemic on 50 U.S. cities (Univ. of Michigan).
{{DEFAULTSORT:Spanish flu
Spanish flu
Pandemics
20th-century epidemics
1918 disease outbreaks
1919 disease outbreaks
1920 disease outbreaks
 This pandemic was known by many different namesŌĆösome old, some newŌĆödepending on place, time, and context. The
This pandemic was known by many different namesŌĆösome old, some newŌĆödepending on place, time, and context. The  Many alternative names are
Many alternative names are  Outside Spain, the disease was soon misnamed 'Spanish influenza'. In a 2 June 1918 ''
Outside Spain, the disease was soon misnamed 'Spanish influenza'. In a 2 June 1918 ''

 The second wave began in the second half of August 1918, probably spreading to
The second wave began in the second half of August 1918, probably spreading to  In the northern hemisphere, fears of a "recurrence" of the flu grew as fall approached. Experts cited the history of past flu epidemics, such as that of 1889ŌĆō1890, to predict that such a recurrence a year later was not unlikely, though not all agreed. In September,
In the northern hemisphere, fears of a "recurrence" of the flu grew as fall approached. Experts cited the history of past flu epidemics, such as that of 1889ŌĆō1890, to predict that such a recurrence a year later was not unlikely, though not all agreed. In September,  The fourth wave in the United States subsided as swiftly as it had appeared, reaching a peak in early February. "An epidemic of considerable proportions marked the early months of 1920", the U.S. Mortality Statistics would later note; according to data at this time, the epidemic resulted in one third as many deaths as the 1918ŌĆō1919 experience. New York City alone reported 6,374 deaths between December 1919 and April 1920, almost twice the number of the first wave in spring 1918. Other US cities including Detroit, Milwaukee, Kansas City, Minneapolis, and St. Louis were hit particularly hard, with death rates higher than all of 1918. The
The fourth wave in the United States subsided as swiftly as it had appeared, reaching a peak in early February. "An epidemic of considerable proportions marked the early months of 1920", the U.S. Mortality Statistics would later note; according to data at this time, the epidemic resulted in one third as many deaths as the 1918ŌĆō1919 experience. New York City alone reported 6,374 deaths between December 1919 and April 1920, almost twice the number of the first wave in spring 1918. Other US cities including Detroit, Milwaukee, Kansas City, Minneapolis, and St. Louis were hit particularly hard, with death rates higher than all of 1918. The  The
The  The majority of the infected experienced only the typical flu symptoms of sore throat, headache, and fever, especially during the first wave. However, during the second wave, the disease was much more serious, often complicated by
The majority of the infected experienced only the typical flu symptoms of sore throat, headache, and fever, especially during the first wave. However, during the second wave, the disease was much more serious, often complicated by  Studies have shown that the immune system of Spanish flu victims was weakened by adverse climate conditions which were particularly unseasonably cold and wet for extended periods of time during the duration of the pandemic. This affected especially WWI troops exposed to incessant rains and lower-than-average temperatures for the duration of the conflict, and especially during the second wave of the pandemic. Ultra-high-resolution climate data combined with highly detailed mortality records analyzed at
Studies have shown that the immune system of Spanish flu victims was weakened by adverse climate conditions which were particularly unseasonably cold and wet for extended periods of time during the duration of the pandemic. This affected especially WWI troops exposed to incessant rains and lower-than-average temperatures for the duration of the conflict, and especially during the second wave of the pandemic. Ultra-high-resolution climate data combined with highly detailed mortality records analyzed at  The Spanish flu infected around 500 million people, about one-third of the world's population. Estimates as to how many infected people died vary greatly, but the flu is regardless considered to be one of the deadliest pandemics in history. An early estimate from 1927 put global mortality at 21.6 million. An estimate from 1991 states that the virus killed between 25 and 39 million people. A 2005 estimate put the death toll at 50 million (about 3% of the global population), and possibly as high as 100 million (more than 5%). However, a 2018 reassessment in the ''
The Spanish flu infected around 500 million people, about one-third of the world's population. Estimates as to how many infected people died vary greatly, but the flu is regardless considered to be one of the deadliest pandemics in history. An early estimate from 1927 put global mortality at 21.6 million. An estimate from 1991 states that the virus killed between 25 and 39 million people. A 2005 estimate put the death toll at 50 million (about 3% of the global population), and possibly as high as 100 million (more than 5%). However, a 2018 reassessment in the '' Even in areas where mortality was low, so many adults were incapacitated that much of everyday life was hampered. Some communities closed all stores or required customers to leave orders outside. There were reports that healthcare workers could not tend the sick nor the gravediggers bury the dead because they too were ill. Mass graves were dug by
Even in areas where mortality was low, so many adults were incapacitated that much of everyday life was hampered. Some communities closed all stores or required customers to leave orders outside. There were reports that healthcare workers could not tend the sick nor the gravediggers bury the dead because they too were ill. Mass graves were dug by  Estimates for the death toll in China have varied widely, a range which reflects the lack of centralized collection of health data at the time due to the Warlord period.
Estimates for the death toll in China have varied widely, a range which reflects the lack of centralized collection of health data at the time due to the Warlord period.  The pandemic mostly killed young adults. In 1918ŌĆō1919, 99% of pandemic influenza deaths in the U.S. occurred in people under 65, and nearly half of deaths were in young adults 20 to 40 years old. In 1920, the mortality rate among people under 65 had decreased sixfold to half the mortality rate of people over 65, but 92% of deaths still occurred in people under 65. This is unusual since influenza is typically most deadly to weak individuals, such as
The pandemic mostly killed young adults. In 1918ŌĆō1919, 99% of pandemic influenza deaths in the U.S. occurred in people under 65, and nearly half of deaths were in young adults 20 to 40 years old. In 1920, the mortality rate among people under 65 had decreased sixfold to half the mortality rate of people over 65, but 92% of deaths still occurred in people under 65. This is unusual since influenza is typically most deadly to weak individuals, such as  Many businesses in the entertainment and service industries suffered losses in revenue, while the healthcare industry reported profit gains. Historian Nancy Bristow has argued that the pandemic, when combined with the increasing number of women attending college, contributed to the success of women in the field of nursing. This was due in part to the failure of medical doctors, who were predominantly men, to contain and prevent the illness. Nursing staff, who were mainly women, celebrated the success of their patient care and did not associate the spread of the disease with their work.
A 2020 study found that US cities that implemented early and extensive non-medical measures (quarantine, etc.) suffered no additional adverse economic effects due to implementing those measures,
however, the validity of this study has been questioned because of the coincidence of WWI and other problems with data reliability.
Many businesses in the entertainment and service industries suffered losses in revenue, while the healthcare industry reported profit gains. Historian Nancy Bristow has argued that the pandemic, when combined with the increasing number of women attending college, contributed to the success of women in the field of nursing. This was due in part to the failure of medical doctors, who were predominantly men, to contain and prevent the illness. Nursing staff, who were mainly women, celebrated the success of their patient care and did not associate the spread of the disease with their work.
A 2020 study found that US cities that implemented early and extensive non-medical measures (quarantine, etc.) suffered no additional adverse economic effects due to implementing those measures,
however, the validity of this study has been questioned because of the coincidence of WWI and other problems with data reliability.
 Despite the high morbidity and mortality rates that resulted from the epidemic, the Spanish flu began to fade from public awareness over the decades until the arrival of news about Avian influenza, bird flu and other pandemics in the 1990s and 2000s. This has led some historians to label the Spanish flu a "forgotten pandemic". However, this label has been challenged by the historian Guy Beiner, who has charted a complex history of social and cultural forgetting, demonstrating how the pandemic was overshadowed by the commemoration of the First World War and mostly neglected in mainstream historiography, yet was remembered in private and local traditions across the globe.
There are various theories of why the Spanish flu was "forgotten". The rapid pace of the pandemic, which killed most of its victims in the United States within less than nine months, resulted in limited media coverage. The general population was familiar with patterns of pandemic disease in the late 19th and early 20th centuries: typhoid, yellow fever, diphtheria, and cholera all occurred near the same time. These outbreaks probably lessened the significance of the influenza pandemic for the public. In some areas, the flu was not reported on, the only mention being that of advertisements for medicines claiming to cure it.
Additionally, the outbreak coincided with the deaths and media focus on the First World War. Another explanation involves the age group affected by the disease. The majority of fatalities, from both the war and the epidemic, were among young adults. The high number of war-related deaths of young adults may have overshadowed the deaths caused by flu.
When people read the obituaries, they saw the war or postwar deaths and the deaths from the influenza side by side. Particularly in Europe, where the war's toll was high, the flu may not have had a tremendous psychological impact or may have seemed an extension of the war's tragedies. The duration of the pandemic and the war could have also played a role. The disease would usually only affect a particular area for a month before leaving. The war, however, had initially been expected to end quickly but lasted for four years by the time the pandemic struck.
Despite the high morbidity and mortality rates that resulted from the epidemic, the Spanish flu began to fade from public awareness over the decades until the arrival of news about Avian influenza, bird flu and other pandemics in the 1990s and 2000s. This has led some historians to label the Spanish flu a "forgotten pandemic". However, this label has been challenged by the historian Guy Beiner, who has charted a complex history of social and cultural forgetting, demonstrating how the pandemic was overshadowed by the commemoration of the First World War and mostly neglected in mainstream historiography, yet was remembered in private and local traditions across the globe.
There are various theories of why the Spanish flu was "forgotten". The rapid pace of the pandemic, which killed most of its victims in the United States within less than nine months, resulted in limited media coverage. The general population was familiar with patterns of pandemic disease in the late 19th and early 20th centuries: typhoid, yellow fever, diphtheria, and cholera all occurred near the same time. These outbreaks probably lessened the significance of the influenza pandemic for the public. In some areas, the flu was not reported on, the only mention being that of advertisements for medicines claiming to cure it.
Additionally, the outbreak coincided with the deaths and media focus on the First World War. Another explanation involves the age group affected by the disease. The majority of fatalities, from both the war and the epidemic, were among young adults. The high number of war-related deaths of young adults may have overshadowed the deaths caused by flu.
When people read the obituaries, they saw the war or postwar deaths and the deaths from the influenza side by side. Particularly in Europe, where the war's toll was high, the flu may not have had a tremendous psychological impact or may have seemed an extension of the war's tragedies. The duration of the pandemic and the war could have also played a role. The disease would usually only affect a particular area for a month before leaving. The war, however, had initially been expected to end quickly but lasted for four years by the time the pandemic struck.
 The origin of the Spanish flu pandemic, and the relationship between the near-simultaneous outbreaks in humans and swine, have been controversial. One hypothesis is that the virus strain originated at Fort Riley, Kansas, in viruses in poultry and swine which the fort bred for food; the soldiers were then sent from Fort Riley around the world, where they spread the disease. Similarities between a reconstruction of the virus and avian viruses, combined with the human pandemic preceding the first reports of influenza in swine, led researchers to conclude the influenza virus jumped directly from birds to humans, and swine caught the disease from humans.
Others have disagreed, and more recent research has suggested the strain may have originated in a nonhuman, mammalian species. An estimated date for its appearance in mammalian hosts has been put at the period 1882ŌĆō1913. This ancestor virus diverged about 1913ŌĆō1915 into two clades (or biological groups each descended from a common ancestor), which gave rise to the classical swine and human H1N1 influenza lineages. The last common ancestor of human strains dates between February 1917 and April 1918. Because pigs are more readily infected with avian influenza viruses than are humans, they were suggested as the original recipients of the virus, passing the virus to humans sometime between 1913 and 1918.
The origin of the Spanish flu pandemic, and the relationship between the near-simultaneous outbreaks in humans and swine, have been controversial. One hypothesis is that the virus strain originated at Fort Riley, Kansas, in viruses in poultry and swine which the fort bred for food; the soldiers were then sent from Fort Riley around the world, where they spread the disease. Similarities between a reconstruction of the virus and avian viruses, combined with the human pandemic preceding the first reports of influenza in swine, led researchers to conclude the influenza virus jumped directly from birds to humans, and swine caught the disease from humans.
Others have disagreed, and more recent research has suggested the strain may have originated in a nonhuman, mammalian species. An estimated date for its appearance in mammalian hosts has been put at the period 1882ŌĆō1913. This ancestor virus diverged about 1913ŌĆō1915 into two clades (or biological groups each descended from a common ancestor), which gave rise to the classical swine and human H1N1 influenza lineages. The last common ancestor of human strains dates between February 1917 and April 1918. Because pigs are more readily infected with avian influenza viruses than are humans, they were suggested as the original recipients of the virus, passing the virus to humans sometime between 1913 and 1918.
 An effort to recreate the Spanish flu strain (a subtype of avian strain H1N1) was a collaboration among the Armed Forces Institute of Pathology, the USDA Agricultural Research Service, ARS Southeast Poultry Research Laboratory, and Mount Sinai School of Medicine in New York City. The effort resulted in the announcement (on 5 October 2005) that the group had successfully determined the virus's genetic sequence, using historic tissue samples recovered by pathologist Johan Hultin from an Inuit female flu victim buried in the Alaskan permafrost and samples preserved from American soldiers Roscoe Vaughan and James Downs.
On 18 January 2007, Kobasa et al. (2007) reported that monkeys (''Macaca fascicularis'') infected with the recreated flu strain exhibited classic symptoms of the 1918 pandemic, and died from cytokine storms ŌĆō an overreaction of the immune system. This may explain why the Spanish flu had its surprising effect on younger, healthier people, as a person with a stronger immune system would potentially have a stronger overreaction.
On 16 September 2008, the body of British politician and diplomat Mark Sykes, Sir Mark Sykes was exhumed to study the RNA of the flu virus in efforts to understand the genetic structure of modern H5N1 bird flu. Sykes had been buried in 1919 in a lead coffin which scientists hoped had helped preserve the virus. The coffin was found to be split and the cadaver badly decomposed; nonetheless, samples of lung and brain tissue were taken.
In December 2008, research by Yoshihiro Kawaoka of the University of Wisconsin linked the presence of three specific genes (termed PA, PB1, and PB2) and a nucleoprotein derived from Spanish flu samples to the ability of the flu virus to invade the lungs and cause pneumonia. The combination triggered similar symptoms in animal testing.
In June 2010, a team at the Mount Sinai School of Medicine reported the 2009 flu pandemic vaccine provided some cross-protection against the Spanish flu pandemic strain.
One of the few things known for certain about influenza in 1918 and for some years after was that it was, except in the laboratory, exclusively a disease of human beings.
In 2013, the AIR Worldwide Research and Modeling Group "characterized the historic 1918 pandemic and estimated the effects of a similar pandemic occurring today using the AIR Pandemic Flu Model". In the model, "a modern-day 'Spanish flu' event would result in additional life insurance losses of between US$15.3ŌĆō27.8 billion in the United States alone", with 188,000ŌĆō337,000 deaths in the United States.
In 2018, Michael Worobey, an evolutionary biology professor at the University of Arizona who is examining the history of the 1918 pandemic, revealed that he obtained tissue slides created by William Rolland, a physician who reported on a respiratory illness likely to be the virus while a pathologist in the British military during World War One. Rolland had authored an article in the ''Lancet (journal), Lancet'' during 1917 about a respiratory illness outbreak beginning in 1916 in ├ētaples, France. Worobey traced recent references to that article to family members who had retained slides that Rolland had prepared during that time. Worobey extracted tissue from the slides to potentially reveal more about the origin of the pathogen.
In 2021 an investigation used the virus sequence to obtain the Hemagglutinin (influenza), Hemagglutinin (HA) antigen and observe the adaptive immunity in 32 survivors of the 1918 flu pandemic, all of them presented seroreactivity and 7 of 8 further tested presented memory B cells able to produce antibodies that bound to the HA antigen highlighting the ability of the immunological memory many decades after.
An effort to recreate the Spanish flu strain (a subtype of avian strain H1N1) was a collaboration among the Armed Forces Institute of Pathology, the USDA Agricultural Research Service, ARS Southeast Poultry Research Laboratory, and Mount Sinai School of Medicine in New York City. The effort resulted in the announcement (on 5 October 2005) that the group had successfully determined the virus's genetic sequence, using historic tissue samples recovered by pathologist Johan Hultin from an Inuit female flu victim buried in the Alaskan permafrost and samples preserved from American soldiers Roscoe Vaughan and James Downs.
On 18 January 2007, Kobasa et al. (2007) reported that monkeys (''Macaca fascicularis'') infected with the recreated flu strain exhibited classic symptoms of the 1918 pandemic, and died from cytokine storms ŌĆō an overreaction of the immune system. This may explain why the Spanish flu had its surprising effect on younger, healthier people, as a person with a stronger immune system would potentially have a stronger overreaction.
On 16 September 2008, the body of British politician and diplomat Mark Sykes, Sir Mark Sykes was exhumed to study the RNA of the flu virus in efforts to understand the genetic structure of modern H5N1 bird flu. Sykes had been buried in 1919 in a lead coffin which scientists hoped had helped preserve the virus. The coffin was found to be split and the cadaver badly decomposed; nonetheless, samples of lung and brain tissue were taken.
In December 2008, research by Yoshihiro Kawaoka of the University of Wisconsin linked the presence of three specific genes (termed PA, PB1, and PB2) and a nucleoprotein derived from Spanish flu samples to the ability of the flu virus to invade the lungs and cause pneumonia. The combination triggered similar symptoms in animal testing.
In June 2010, a team at the Mount Sinai School of Medicine reported the 2009 flu pandemic vaccine provided some cross-protection against the Spanish flu pandemic strain.
One of the few things known for certain about influenza in 1918 and for some years after was that it was, except in the laboratory, exclusively a disease of human beings.
In 2013, the AIR Worldwide Research and Modeling Group "characterized the historic 1918 pandemic and estimated the effects of a similar pandemic occurring today using the AIR Pandemic Flu Model". In the model, "a modern-day 'Spanish flu' event would result in additional life insurance losses of between US$15.3ŌĆō27.8 billion in the United States alone", with 188,000ŌĆō337,000 deaths in the United States.
In 2018, Michael Worobey, an evolutionary biology professor at the University of Arizona who is examining the history of the 1918 pandemic, revealed that he obtained tissue slides created by William Rolland, a physician who reported on a respiratory illness likely to be the virus while a pathologist in the British military during World War One. Rolland had authored an article in the ''Lancet (journal), Lancet'' during 1917 about a respiratory illness outbreak beginning in 1916 in ├ētaples, France. Worobey traced recent references to that article to family members who had retained slides that Rolland had prepared during that time. Worobey extracted tissue from the slides to potentially reveal more about the origin of the pathogen.
In 2021 an investigation used the virus sequence to obtain the Hemagglutinin (influenza), Hemagglutinin (HA) antigen and observe the adaptive immunity in 32 survivors of the 1918 flu pandemic, all of them presented seroreactivity and 7 of 8 further tested presented memory B cells able to produce antibodies that bound to the HA antigen highlighting the ability of the immunological memory many decades after.