Immunoglobulin E on:
[Wikipedia]
[Google]
[Amazon]
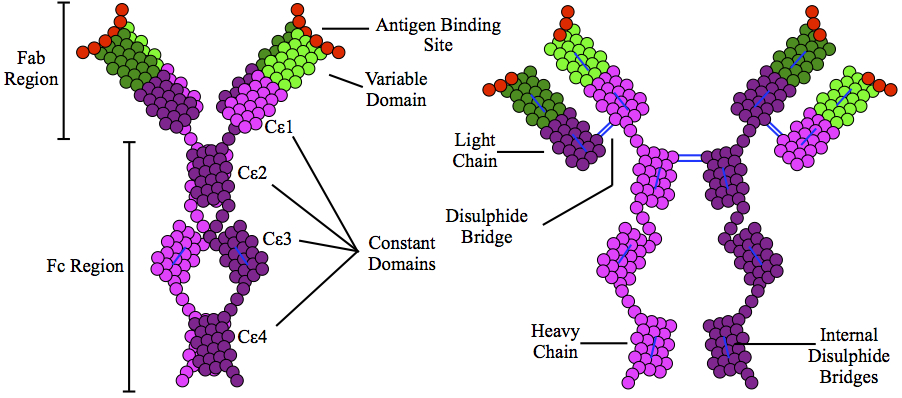 Immunoglobulin E (IgE) is a type of
Immunoglobulin E (IgE) is a type of
 Immunoglobulin E (IgE) is a type of
Immunoglobulin E (IgE) is a type of antibody
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the ...
(or immunoglobulin
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the ...
(Ig) " isotype") that has been found only in mammal
Mammals () are a group of vertebrate animals constituting the class Mammalia (), characterized by the presence of mammary glands which in females produce milk for feeding (nursing) their young, a neocortex (a region of the brain), fur or ...
s. IgE is synthesised by plasma cell
Plasma cells, also called plasma B cells or effector B cells, are white blood cells that originate in the lymphoid organs as B lymphocytes and secrete large quantities of proteins called antibodies in response to being presented specific substan ...
s. Monomers of IgE consist of two heavy chains (ε chain) and two light chains, with the ε chain containing four Ig-like constant domains (Cε1–Cε4). IgE is thought to be an important part of the immune response
An immune response is a reaction which occurs within an organism for the purpose of defending against foreign invaders. These invaders include a wide variety of different microorganisms including viruses, bacteria, parasites, and fungi which could ...
against infection by certain parasitic worm
Parasitic worms, also known as helminths, are large macroparasites; adults can generally be seen with the naked eye. Many are intestinal worms that are soil-transmitted and infect the gastrointestinal tract. Other parasitic worms such as sc ...
s, including ''Schistosoma mansoni
A paired couple of ''Schistosoma mansoni''.
''Schistosoma mansoni'' is a water-borne parasite of humans, and belongs to the group of blood flukes (''Schistosoma''). The adult lives in the blood vessels ( mesenteric veins) near the human inte ...
'', ''Trichinella spiralis
''Trichinella spiralis'' is a viviparous nematode parasite, occurring in rodents, pigs, bears, hyenas and humans, and is responsible for the disease trichinosis. It is sometimes referred to as the "pork worm" due to it being typically encounte ...
'', and ''Fasciola hepatica
''Fasciola hepatica'', also known as the common liver fluke or sheep liver fluke, is a parasitic trematode (fluke or flatworm, a type of helminth) of the class Trematoda, phylum Platyhelminthes. It infects the livers of various mammals, inc ...
''. IgE is also utilized during immune defense against certain protozoa
Protozoa (singular: protozoan or protozoon; alternative plural: protozoans) are a group of single-celled eukaryotes, either free-living or parasitic, that feed on organic matter such as other microorganisms or organic tissues and debris. Histo ...
n parasite
Parasitism is a close relationship between species, where one organism, the parasite, lives on or inside another organism, the host, causing it some harm, and is adapted structurally to this way of life. The entomologist E. O. Wilson has ...
s such as ''Plasmodium falciparum
''Plasmodium falciparum'' is a Unicellular organism, unicellular protozoan parasite of humans, and the deadliest species of ''Plasmodium'' that causes malaria in humans. The parasite is transmitted through the bite of a female ''Anopheles'' mosqu ...
''. IgE may have evolved as a defense to protect against venoms.
IgE also has an essential role in type I hypersensitivity
Type I hypersensitivity (or immediate hypersensitivity), in the Gell and Coombs classification of allergic reactions, is an allergic reaction provoked by re-exposure to a specific type of antigen referred to as an allergen. Type I is distinct fro ...
, which manifests in various allergic diseases, such as allergic asthma
Asthma is a long-term inflammatory disease of the airways of the lungs. It is characterized by variable and recurring symptoms, reversible airflow obstruction, and easily triggered bronchospasms. Symptoms include episodes of wheezing, cou ...
, most types of sinusitis
Sinusitis, also known as rhinosinusitis, is inflammation of the mucous membranes that line the sinuses resulting in symptoms that may include thick nasal mucus, a plugged nose, and facial pain. Other signs and symptoms may include fever, head ...
, allergic rhinitis
Allergic rhinitis, of which the seasonal type is called hay fever, is a type of inflammation in the nose that occurs when the immune system overreacts to allergens in the air. Signs and symptoms include a runny or stuffy nose, sneezing, red, i ...
, food allergies, and specific types of chronic urticaria
Hives, also known as urticaria, is a kind of skin rash with red, raised, itchy bumps. Hives may burn or sting. The patches of rash may appear on different body parts, with variable duration from minutes to days, and does not leave any long-lasti ...
and atopic dermatitis
Atopic dermatitis (AD), also known as atopic eczema, is a long-term type of inflammation of the skin (dermatitis). It results in puritis, itchy, red, swollen, and cracked skin. Clear fluid may come from the affected areas, which often thickens o ...
. IgE also plays a pivotal role in responses to allergens, such as: anaphylactic
Anaphylaxis is a serious, potentially fatal allergic reaction and medical emergency that is rapid in onset and requires immediate medical attention regardless of use of emergency medication on site. It typically causes more than one of the follo ...
reactions to drugs, bee stings, and antigen preparations used in desensitization immunotherapy
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as ''activation immunotherapies,'' while immunotherap ...
.
Although IgE is typically the least abundant isotype— blood serum IgE levels in a normal ("non-atopic
Atopy is the tendency to produce an exaggerated immunoglobulin E (IgE) immune response to otherwise harmless substances in the environment. Allergic diseases are clinical manifestations of such inappropriate, atopic responses.
Atopy may have a ...
") individual are only 0.05% of the Ig concentration, compared to 75% for the IgGs at 10 mg/ml, which are the isotypes responsible for most of the classical adaptive immune response—it is capable of triggering anaphylaxis
Anaphylaxis is a serious, potentially fatal allergic reaction and medical emergency that is rapid in onset and requires immediate medical attention regardless of use of emergency medication on site. It typically causes more than one of the follow ...
, one of the most rapid and severe immunological reactions.
__TOC__
Discovery
IgE was simultaneously discovered in 1966 and 1967 by two independent groups:Kimishige Ishizaka
was a Japanese immunologist who, with his wife Teruko Ishizaka, discovered the antibody class Immunoglobulin E (IgE) in 1966–1967. Their work was regarded as a major breakthrough in the understanding of allergy. He was awarded the 1973 Gair ...
and his wife Teruko Ishizaka
was a Japanese scientist and immunologist who along with her husband Kimishige Ishizaka discovered the antibody class Immunoglobulin E (IgE) in 1966. Their work was regarded as a major breakthrough in the understanding of allergy, and for thi ...
at the Children's Asthma Research Institute and Hospital in Denver
Denver () is a consolidated city and county, the capital, and most populous city of the U.S. state of Colorado. Its population was 715,522 at the 2020 census, a 19.22% increase since 2010. It is the 19th-most populous city in the Unit ...
, Colorado
Colorado (, other variants) is a state in the Mountain West subregion of the Western United States. It encompasses most of the Southern Rocky Mountains, as well as the northeastern portion of the Colorado Plateau and the western edge of t ...
, and by Gunnar Johansson and in Uppsala
Uppsala (, or all ending in , ; archaically spelled ''Upsala'') is the county seat of Uppsala County and the List of urban areas in Sweden by population, fourth-largest city in Sweden, after Stockholm, Gothenburg, and Malmö. It had 177,074 inha ...
, Sweden
Sweden, formally the Kingdom of Sweden,The United Nations Group of Experts on Geographical Names states that the country's formal name is the Kingdom of SwedenUNGEGN World Geographical Names, Sweden./ref> is a Nordic country located on ...
. Their joint paper was published in April 1969.
Receptors
IgE primes the IgE-mediated allergic response by binding to Fc receptors found on the surface ofmast cell
A mast cell (also known as a mastocyte or a labrocyte) is a resident cell of connective tissue that contains many granules rich in histamine and heparin. Specifically, it is a type of granulocyte derived from the myeloid stem cell that is a par ...
s and basophil
Basophils are a type of white blood cell. Basophils are the least common type of granulocyte, representing about 0.5% to 1% of circulating white blood cells. However, they are the largest type of granulocyte. They are responsible for inflammator ...
s. Fc receptors are also found on eosinophil
Eosinophils, sometimes called eosinophiles or, less commonly, acidophils, are a variety of white blood cells (WBCs) and one of the immune system components responsible for combating multicellular parasites and certain infections in vertebrates. A ...
s, monocyte
Monocytes are a type of leukocyte or white blood cell. They are the largest type of leukocyte in blood and can differentiate into macrophages and conventional dendritic cells. As a part of the vertebrate innate immune system monocytes also inf ...
s, macrophage
Macrophages (abbreviated as M φ, MΦ or MP) ( el, large eaters, from Greek ''μακρός'' (') = large, ''φαγεῖν'' (') = to eat) are a type of white blood cell of the immune system that engulfs and digests pathogens, such as cancer cel ...
s and platelet
Platelets, also called thrombocytes (from Greek θρόμβος, "clot" and κύτος, "cell"), are a component of blood whose function (along with the coagulation factors) is to react to bleeding from blood vessel injury by clumping, thereby ini ...
s in humans. There are two types of Fcε receptors:
* FcεRI
The high-affinity IgE receptor, also known as FcεRI, or Fc epsilon RI, is the high- affinity receptor for the Fc region of immunoglobulin E (IgE), an antibody isotype involved in the allergy disorder and parasites immunity. FcεRI is a tetra ...
(type I Fcε receptor), the high-affinity IgE receptor
* FcεRII
CD23, also known as Fc epsilon RII, or FcεRII, is the "low-affinity" receptor for IgE, an antibody isotype involved in allergy and resistance to parasites, and is important in regulation of IgE levels. Unlike many of the antibody receptors, CD23 ...
(type II Fcε receptor), also known as CD23, the low-affinity IgE receptor
IgE can upregulate the expression of both types of Fcε receptors. FcεRI is expressed on mast cells, basophils, and the antigen-presenting dendritic cells in both mice and humans. Binding of antigen
In immunology, an antigen (Ag) is a molecule or molecular structure or any foreign particulate matter or a pollen grain that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response. ...
s to IgE already bound by the FcεRI on mast cells causes cross-linking of the bound IgE and the aggregation of the underlying FcεRI, leading to degranulation (the release of mediators) and the secretion of several types of type 2 cytokine
Cytokines are a broad and loose category of small proteins (~5–25 kDa) important in cell signaling. Cytokines are peptides and cannot cross the lipid bilayer of cells to enter the cytoplasm. Cytokines have been shown to be involved in autocrin ...
s like interleukin
Interleukins (ILs) are a group of cytokines (secreted proteins and signal molecules) that are expressed and secreted by white blood cells (leukocytes) as well as some other body cells. The human genome encodes more than 50 interleukins and related ...
(IL)-3 and stem cell factor (SCF), which both help the mast cells survive and accumulate in tissue, and IL-4, IL-5, IL-13, and IL-33
Interleukin 33 (IL-33) is a protein that in humans is encoded by the ''IL33'' gene.
Interleukin 33 is a member of the IL-1 family that potently drives production of T helper-2 (Th2)-associated cytokines (e.g., IL-4). IL33 is a ligand for ST2 ...
, which in turn activate group 2-innate lymphoid cells ( ILC2 or natural helper cells). Basophils share a common haemopoietic progenitor with mast cells; upon the cross-linking of their surface bound IgE by antigens, also release type 2 cytokines, including IL-4 and IL-13, and other inflammatory mediators. The low-affinity receptor (FcεRII) is always expressed on B cell
B cells, also known as B lymphocytes, are a type of white blood cell of the lymphocyte subtype. They function in the humoral immunity component of the adaptive immune system. B cells produce antibody molecules which may be either secreted or ...
s; but IL-4 can induce its expression on the surfaces of macrophages, eosinophils, platelets, and some T cell
A T cell is a type of lymphocyte. T cells are one of the important white blood cells of the immune system and play a central role in the adaptive immune response. T cells can be distinguished from other lymphocytes by the presence of a T-cell r ...
s.
Function
Parasite hypothesis
The IgE isotype has co-evolved with basophils and mast cells in the defence against parasites like helminths (like Schistosoma) but may be also effective in bacterial infections. Epidemiological research shows that IgE level is increased when infected by ''Schistosoma mansoni'', ''Necator americanus
''Necator americanus'' is a species of hookworm (a type of helminth) commonly known as the New World hookworm. Like other hookworms, it is a member of the phylum Nematoda. It is an obligatory parasitic nematode that lives in the small intestine ...
'', and nematode
The nematodes ( or grc-gre, Νηματώδη; la, Nematoda) or roundworms constitute the phylum Nematoda (also called Nemathelminthes), with plant-Parasitism, parasitic nematodes also known as eelworms. They are a diverse animal phylum inhab ...
s in humans. It is most likely beneficial in removal of hookworms from the lung.
Toxin hypothesis of allergic disease
In 1981Margie Profet
Margaret J. "Margie" Profet (born August 7, 1958) is an American evolutionary biologist with no formal biology training who created a decade-long controversy when she published her findings on the role of Darwinian evolution in menstruation, all ...
suggested that allergic reactions have evolved as a last line of defense to protect against venom
Venom or zootoxin is a type of toxin produced by an animal that is actively delivered through a wound by means of a bite, sting, or similar action. The toxin is delivered through a specially evolved ''venom apparatus'', such as fangs or a sti ...
s. Although controversial at the time, new work supports some of Profet’s thoughts on the adaptive role of allergies as a defense against noxious toxins.
In 2013 it emerged that IgE-antibodies play an essential role in acquired resistance to honey bee
A honey bee (also spelled honeybee) is a eusocial flying insect within the genus ''Apis'' of the bee clade, all native to Afro-Eurasia. After bees spread naturally throughout Africa and Eurasia, humans became responsible for the current co ...
and Russell's viper
Russell's viper (''Daboia russelii''), is a venomous snake in the family Viperidae native to the Indian subcontinent and one of the big four snakes in India. It was described in 1797 by George Shaw and Frederick Polydore Nodder, and named af ...
venoms. The authors concluded that "a small dose of bee venom conferred immunity to a much larger, fatal dose" and "this kind of venom-specific, IgE-associated, adaptive immune response developed, at least in evolutionary terms, to protect the host against potentially toxic amounts of venom, such as would happen if the animal encountered a whole nest of bees, or in the event of a snakebite". The major allergen of bee venom (phospholipase A2
The enzyme phospholipase A2 (EC 3.1.1.4, PLA2, systematic name phosphatidylcholine 2-acylhydrolase) catalyse the cleavage of fatty acids in position 2 of phospholipids, hydrolyzing the bond between the second fatty acid “tail” and the glyce ...
) induces a Th2 immune responses, associated with production of IgE antibodies, which may "increase the resistance of mice to challenge with potentially lethal doses".
Cancer
Although it is not yet well understood, IgE may play an important role in the immune system's recognition ofcancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
, in which the stimulation of a strong cytotoxic response against cells displaying only small amounts of early cancer markers would be beneficial. If this were the case, anti-IgE treatments such as omalizumab
Omalizumab, sold under the brand name Xolair, is a medication used to treat asthma, nasal polyps, and urticaria (hives).
Omalizumab is a recombinant DNA-derived humanized IgG1k monoclonal antibody that specifically binds to free human immunoglo ...
(for allergies) might have some undesirable side effects. However, a recent study, which was performed based on pooled analysis using comprehensive data from 67 phase I to IV clinical trials of omalizumab in various indications, concluded that a causal relationship between omalizumab therapy and malignancy is unlikely.
Role in disease
Atopic
Atopy is the tendency to produce an exaggerated immunoglobulin E (IgE) immune response to otherwise harmless substances in the environment. Allergic diseases are clinical manifestations of such inappropriate, atopic responses.
Atopy may have a ...
individuals can have up to ten times the normal level of IgE in their blood (as do sufferers of hyper-IgE syndrome). However, this may not be a requirement for symptoms to occur as has been seen in asthmatics with normal IgE levels in their blood—recent research has shown that IgE production can occur locally in the nasal mucosa.
IgE that can specifically recognise an allergen
An allergen is a type of antigen that produces an abnormally vigorous immune response in which the immune system fights off a perceived threat that would otherwise be harmless to the body. Such reactions are called allergies.
In technical terms ...
(typically this is a protein, such as dust mite
House dust mites (HDM, or simply dust mites) are various species of acariform mites belonging to the family Pyroglyphidae that are found in association with dust in dwellings. They are known for causing allergies.
Biology
Species
The curren ...
Der p 1, cat Fel d 1
Fel d 1 is a secretoglobin protein complex that, in cats, is encoded by the ''CH1'' (chain 1/Fel d 1-A) and ''CH2'' (chain 2/Fel d 1-B) genes.
Among cats, Fel d 1 is produced largely in their saliva and by the sebaceous glands located in the ...
, grass or ragweed
Ragweeds are flowering plants in the genus ''Ambrosia'' in the aster family, Asteraceae. They are distributed in the tropical and subtropical regions of the Americas, especially North America,receptor
Receptor may refer to:
* Sensory receptor, in physiology, any structure which, on receiving environmental stimuli, produces an informative nerve impulse
*Receptor (biochemistry), in biochemistry, a protein molecule that receives and responds to a ...
FcεRI so that basophils
Basophils are a type of white blood cell. Basophils are the least common type of granulocyte, representing about 0.5% to 1% of circulating white blood cells. However, they are the largest type of granulocyte. They are responsible for inflammator ...
and mast cells
A mast cell (also known as a mastocyte or a labrocyte) is a resident cell of connective tissue that contains many granules rich in histamine and heparin. Specifically, it is a type of granulocyte derived from the myeloid stem cell that is a par ...
, capable of mediating inflammatory reactions, become "primed", ready to release chemicals like histamine
Histamine is an organic nitrogenous compound involved in local immune responses, as well as regulating physiological functions in the gut and acting as a neurotransmitter for the brain, spinal cord, and uterus. Since histamine was discovered ...
, leukotriene
Leukotrienes are a family of eicosanoid inflammatory mediators produced in leukocytes by the oxidation of arachidonic acid (AA) and the essential fatty acid eicosapentaenoic acid (EPA) by the enzyme arachidonate 5-lipoxygenase.
Leukotrienes ...
s, and certain interleukins. These chemicals cause many of the symptoms we associate with allergy, such as airway constriction in asthma
Asthma is a long-term inflammatory disease of the airways of the lungs. It is characterized by variable and recurring symptoms, reversible airflow obstruction, and easily triggered bronchospasms. Symptoms include episodes of wheezing, cou ...
, local inflammation in eczema
Dermatitis is inflammation of the skin, typically characterized by itchiness, redness and a rash. In cases of short duration, there may be small blisters, while in long-term cases the skin may become thickened. The area of skin involved can ...
, increased mucus
Mucus ( ) is a slippery aqueous secretion produced by, and covering, mucous membranes. It is typically produced from cells found in mucous glands, although it may also originate from mixed glands, which contain both serous and mucous cells. It is ...
secretion in allergic rhinitis
Allergic rhinitis, of which the seasonal type is called hay fever, is a type of inflammation in the nose that occurs when the immune system overreacts to allergens in the air. Signs and symptoms include a runny or stuffy nose, sneezing, red, i ...
, and increased vascular permeability, it is presumed, to allow other immune cells to gain access to tissues, but which can lead to a potentially fatal drop in blood pressure as in anaphylaxis
Anaphylaxis is a serious, potentially fatal allergic reaction and medical emergency that is rapid in onset and requires immediate medical attention regardless of use of emergency medication on site. It typically causes more than one of the follow ...
.
IgE is known to be elevated in various autoimmune disorders such as SLE
Lupus, technically known as systemic lupus erythematosus (SLE), is an autoimmune disease in which the body's immune system mistakenly attacks healthy tissue in many parts of the body. Symptoms vary among people and may be mild to severe. Commo ...
, rheumatoid arthritis
Rheumatoid arthritis (RA) is a long-term autoimmune disorder that primarily affects joints. It typically results in warm, swollen, and painful joints. Pain and stiffness often worsen following rest. Most commonly, the wrist and hands are involv ...
(RA), and psoriasis
Psoriasis is a long-lasting, noncontagious autoimmune disease characterized by raised areas of abnormal skin. These areas are red, pink, or purple, dry, itchy, and scaly. Psoriasis varies in severity from small, localized patches to complete ...
, and is theorized to be of pathogenetic importance in SLE and RA by eliciting a hypersensitivity reaction.
Regulation of IgE levels through control of B cell differentiation to antibody-secreting plasma cells
Plasma cells, also called plasma B cells or effector B cells, are white blood cells that originate in the lymphoid organs as B lymphocytes and secrete large quantities of proteins called antibodies in response to being presented specific substan ...
is thought to involve the "low-affinity" receptor FcεRII, or CD23
CD23, also known as Fc epsilon RII, or FcεRII, is the "low-affinity" receptor for IgE, an antibody isotype involved in allergy and resistance to parasites, and is important in regulation of IgE levels. Unlike many of the antibody receptors, CD23 ...
. CD23
CD23, also known as Fc epsilon RII, or FcεRII, is the "low-affinity" receptor for IgE, an antibody isotype involved in allergy and resistance to parasites, and is important in regulation of IgE levels. Unlike many of the antibody receptors, CD23 ...
may also allow facilitated antigen presentation, an IgE-dependent mechanism whereby B cells
B cells, also known as B lymphocytes, are a type of white blood cell of the lymphocyte subtype. They function in the humoral immunity component of the adaptive immune system. B cells produce antibody molecules which may be either secreted or ...
expressing CD23
CD23, also known as Fc epsilon RII, or FcεRII, is the "low-affinity" receptor for IgE, an antibody isotype involved in allergy and resistance to parasites, and is important in regulation of IgE levels. Unlike many of the antibody receptors, CD23 ...
are able to present allergen to (and stimulate) specific T helper cells
The T helper cells (Th cells), also known as CD4+ cells or CD4-positive cells, are a type of T cell that play an important role in the adaptive immune system. They aid the activity of other immune cells by releasing cytokines. They are considere ...
, causing the perpetuation of a Th2 response, one of the hallmarks of which is the production of more antibodies.
Role in diagnosis
Diagnosis of allergy is most often done by reviewing a person's medical history and finding a positive result for the presence of allergen specific IgE when conducting a skin or blood test. Specific IgE testing is the proven test for allergy detection; evidence does not show that indiscriminate IgE testing or testing for immunoglobulin G (IgG) can support allergy diagnosis.Drugs targeting the IgE pathway
Currently, allergic diseases and asthma are usually treated with one or more of the following drugs: (1)antihistamines
Antihistamines are drugs which treat allergic rhinitis, common cold, influenza, and other allergies. Typically, people take antihistamines as an inexpensive, generic (not patented) drug that can be bought without a prescription and provides re ...
and antileukotriene
An antileukotriene, also known as leukotriene modifier and leukotriene receptor antagonist, is a medication which functions as a leukotriene-related enzyme inhibitor (arachidonate 5-lipoxygenase) or leukotriene receptor antagonist (cysteinyl leukot ...
s, which antagonize the inflammatory mediators histamine and leukotrienes, (2) local or systemic (oral or injectable) corticosteroids, which suppress a broad spectrum of inflammatory mechanisms, (3) short or long-acting bronchodilators
A bronchodilator or broncholytic (although the latter occasionally includes secretory inhibition as well) is a substance that dilates the bronchi and bronchioles, decreasing resistance in the respiratory airway and increasing airflow to the lungs ...
, which relax smooth muscle of constricted airway in asthma, or (4) mast cell stabilizer
Mast cell stabilizers are medications used to prevent or control certain allergic disorders. They block mast cell degranulation, stabilizing the cell and thereby preventing the release of histamine and related mediators. One suspected pharmaco ...
s, which inhibit the degranulation of mast cells that is normally triggered by IgE-binding at FcεRI
The high-affinity IgE receptor, also known as FcεRI, or Fc epsilon RI, is the high- affinity receptor for the Fc region of immunoglobulin E (IgE), an antibody isotype involved in the allergy disorder and parasites immunity. FcεRI is a tetra ...
. Long-term uses of systemic corticosteroids are known to cause many serious side effects and are advisable to avoid, if alternative therapies are available.
IgE, the IgE synthesis pathway, and the IgE-mediated allergic/inflammatory pathway are all important targets in intervening with the pathological processes of allergy, asthma, and other IgE-mediated diseases. The B lymphocyte differentiation and maturation pathway that eventually generate IgE-secreting plasma cells go through the intermediate steps of IgE-expressing B lymphoblasts and involves the interaction with IgE-expressing memory B cells. Tanox
Tanox was a biopharmaceutical company based in Houston, Texas. The company was founded by two biomedical research scientists, Nancy T. Chang and Tse Wen Chang in March 1986 with $250,000, which was a large part of their family savings at that ...
, a biotech company based in Houston, Texas, proposed in 1987 that by targeting membrane-bound IgE (mIgE) on B lymphoblast and memory B cells, those cells can be lysed or down-regulated, thus achieving the inhibition of the production of antigen-specific IgE and hence a shift of immune balance toward non-IgE mechanisms. Two approaches targeting the IgE pathway were evolved and both are in active development. In the first approach, the anti-IgE
Anti-immunoglobulin Antibody, antibodies are defined as a protein that detects other antibodies from an organism. Specifically, anti-immunoglobulin antibodies are created by B cell, B-cells as antibodies to bind to other immunoglobulins. Immunoglob ...
antibody drug omalizumab
Omalizumab, sold under the brand name Xolair, is a medication used to treat asthma, nasal polyps, and urticaria (hives).
Omalizumab is a recombinant DNA-derived humanized IgG1k monoclonal antibody that specifically binds to free human immunoglo ...
(trade name Xolair
Omalizumab, sold under the brand name Xolair, is a medication used to treat asthma, nasal polyps, and urticaria (hives).
Omalizumab is a recombinant DNA-derived humanized IgG1k monoclonal antibody that specifically binds to free human immunoglo ...
) recognises IgE not bound to its receptors and is used to neutralise or mop-up existing IgE and prevent it from binding to the receptors on mast cells and basophils. Xolair has been approved in many countries for treating severe, persistent allergic asthma. It has also been approved in March 2014 in the European Union and the U. S. for treating chronic spontaneous urticaria
Hives, also known as urticaria, is a kind of skin rash with red, raised, itchy bumps. Hives may burn or sting. The patches of rash may appear on different body parts, with variable duration from minutes to days, and does not leave any long-last ...
, which cannot be adequately treated with H1-antihistamines
Antihistamines are drugs which treat allergic rhinitis, common cold, influenza, and other allergies. Typically, people take antihistamines as an inexpensive, generic (not patented) drug that can be bought without a prescription and provides re ...
. In the second approach, antibodies specific for a domain of 52 amino acid residues, referred to as CεmX or M1’ (M1 prime), present only on human mIgE on B cells and not on free, soluble IgE, have been prepared and are under clinical development for the treatment of allergy and asthma. An anti-M1’ humanized antibody, quilizumab
Quilizumab (INN) is a humanized monoclonal antibody designed for the treatment of asthma
Asthma is a long-term inflammatory disease of the airways of the lungs. It is characterized by variable and recurring symptoms, reversible airflow o ...
, is in phase IIb clinical trial.
In 2002, researchers at the Randall Division of Cell and Molecular Biophysics
The Randall Division of Cell and Molecular Biophysics (the Randall) is a research institute of King's College London located in London United Kingdom. It is a centre for study in allergy and asthma; muscle signalling and development; structura ...
determined the structure of IgE. Understanding of this structure (which is atypical of other isotypes in that it is highly bent and asymmetric) and of the interaction of IgE with receptor FcεRI will enable development of a new generation of allergy drugs that seek to interfere with the IgE-receptor interaction. It may be possible to design treatments cheaper than monoclonal antibodies (for instance, small molecule drugs) that use a similar approach to inhibit binding of IgE to its receptor.
References
{{Portal bar, Biology, Medicine Glycoproteins Antibodies