The immune system is a network of
biological processes that protects an
organism
In biology, an organism () is any living system that functions as an individual entity. All organisms are composed of cells (cell theory). Organisms are classified by taxonomy into groups such as multicellular animals, plants, and ...
from
diseases. It detects and responds to a wide variety of
pathogen
In biology, a pathogen ( el, πάθος, "suffering", "passion" and , "producer of") in the oldest and broadest sense, is any organism or agent that can produce disease. A pathogen may also be referred to as an infectious agent, or simply a germ ...
s, from
virus
A virus is a submicroscopic infectious agent that replicates only inside the living cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and archaea.
Since Dmitri Ivanovsk ...
es to
parasitic worm
Parasitic worms, also known as helminths, are large macroparasites; adults can generally be seen with the naked eye. Many are intestinal worms that are soil-transmitted and infect the gastrointestinal tract. Other parasitic worms such as sc ...
s, as well as
cancer cells and objects such as wood
splinter
A splinter (also known as a sliver) is a fragment of a larger object, or a foreign body that penetrates or is purposely injected into a body. The foreign body must be lodged inside tissue to be considered a splinter. Splinters may cause initia ...
s, distinguishing them from the organism's own healthy
tissue. Many species have two major subsystems of the immune system. The
innate immune system provides a preconfigured response to broad groups of situations and stimuli. The
adaptive immune system
The adaptive immune system, also known as the acquired immune system, is a subsystem of the immune system that is composed of specialized, systemic cells and processes that eliminate pathogens or prevent their growth. The acquired immune system ...
provides a tailored response to each stimulus by learning to recognize molecules it has previously encountered. Both use
molecules and
cells to perform their functions.
Nearly all organisms have some kind of immune system.
Bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of prokaryotic microorganisms. Typically a few micrometr ...
have a rudimentary immune system in the form of
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
s that protect against
virus
A virus is a submicroscopic infectious agent that replicates only inside the living cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and archaea.
Since Dmitri Ivanovsk ...
infections. Other basic immune mechanisms evolved in ancient
plants and animals
Plants and Animals are a Canadian indie-rock band from Montreal (featuring two members originally from Nova Scotia) which comprises guitarist-vocalists Warren Spicer and Nic Basque and drummer-vocalist Matthew Woody Woodley. The trio began playin ...
and remain in their modern descendants. These mechanisms include
phagocytosis
Phagocytosis () is the process by which a cell uses its plasma membrane to engulf a large particle (≥ 0.5 μm), giving rise to an internal compartment called the phagosome. It is one type of endocytosis. A cell that performs phagocytosis is ...
,
antimicrobial peptides called
defensins, and the
complement system.
Jawed vertebrate
Gnathostomata (; from Greek: (') "jaw" + (') "mouth") are the jawed vertebrates. Gnathostome diversity comprises roughly 60,000 species, which accounts for 99% of all living vertebrates, including humans. In addition to opposing jaws, living ...
s, including humans, have even more sophisticated defense mechanisms, including the ability to adapt to recognize pathogens more efficiently. Adaptive (or acquired) immunity creates an
immunological memory
Immunological memory is the ability of the immune system to quickly and specifically recognize an antigen that the body has previously encountered and initiate a corresponding immune response. Generally, these are secondary, tertiary and other subs ...
leading to an enhanced response to subsequent encounters with that same pathogen. This process of acquired immunity is the basis of
vaccination
Vaccination is the administration of a vaccine to help the immune system develop immunity from a disease. Vaccines contain a microorganism or virus in a weakened, live or killed state, or proteins or toxins from the organism. In stimulating ...
.
Dysfunction of the immune system can cause
autoimmune diseases,
inflammatory diseases
Inflammation (from la, inflammatio) is part of the complex biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or irritants, and is a protective response involving immune cells, blood vessels, and molecu ...
and
cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
.
Immunodeficiency occurs when the immune system is less active than normal, resulting in recurring and life-threatening infections. In humans, immunodeficiency can be the result of a
genetic disease
A genetic disorder is a health problem caused by one or more abnormalities in the genome. It can be caused by a mutation in a single gene (monogenic) or multiple genes (polygenic) or by a chromosomal abnormality. Although polygenic disorders ...
such as
severe combined immunodeficiency
Severe combined immunodeficiency (SCID), also known as Swiss-type agammaglobulinemia, is a rare genetic disorder characterized by the disturbed development of functional T cells and B cells caused by numerous genetic mutations that result in diffe ...
, acquired conditions such as
HIV
The human immunodeficiency viruses (HIV) are two species of ''Lentivirus'' (a subgroup of retrovirus) that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the immune ...
/
AIDS, or the use of
immunosuppressive medication
Immunosuppressive drugs, also known as immunosuppressive agents, immunosuppressants and antirejection medications, are drugs that inhibit or prevent activity of the immune system.
Classification
Immunosuppressive drugs can be classified into ...
.
Autoimmunity
In immunology, autoimmunity is the system of immune responses of an organism against its own healthy cells, tissues and other normal body constituents. Any disease resulting from this type of immune response is termed an "autoimmune disease". ...
results from a hyperactive immune system attacking normal tissues as if they were foreign organisms. Common autoimmune diseases include
Hashimoto's thyroiditis
Hashimoto's thyroiditis, also known as chronic lymphocytic thyroiditis and Hashimoto's disease, is an autoimmune disease in which the thyroid gland is gradually destroyed. Early on, symptoms may not be noticed. Over time, the thyroid may enlarg ...
,
rheumatoid arthritis
Rheumatoid arthritis (RA) is a long-term autoimmune disorder that primarily affects joints. It typically results in warm, swollen, and painful joints. Pain and stiffness often worsen following rest. Most commonly, the wrist and hands are invol ...
,
diabetes mellitus type 1
Type 1 diabetes (T1D), formerly known as juvenile diabetes, is an autoimmune disease that originates when cells that make insulin (beta cells) are destroyed by the immune system. Insulin is a hormone required for the cells to use blood sugar for ...
, and
systemic lupus erythematosus.
Immunology
Immunology is a branch of medicineImmunology for Medical Students, Roderick Nairn, Matthew Helbert, Mosby, 2007 and biology that covers the medical study of immune systems in humans, animals, plants and sapient species. In such we can see the ...
covers the study of all aspects of the immune system.
Layered defense
The immune system protects its host from
infection
An infection is the invasion of tissues by pathogens, their multiplication, and the reaction of host tissues to the infectious agent and the toxins they produce. An infectious disease, also known as a transmissible disease or communicable dis ...
with layered defenses of increasing specificity. Physical barriers prevent pathogens such as
bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of prokaryotic microorganisms. Typically a few micrometr ...
and
virus
A virus is a submicroscopic infectious agent that replicates only inside the living cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and archaea.
Since Dmitri Ivanovsk ...
es from entering the organism. If a pathogen breaches these barriers, the
innate immune system provides an immediate, but non-specific response. Innate immune systems are found in all
animal
Animals are multicellular, eukaryotic organisms in the Kingdom (biology), biological kingdom Animalia. With few exceptions, animals Heterotroph, consume organic material, Cellular respiration#Aerobic respiration, breathe oxygen, are Motilit ...
s.
If pathogens successfully evade the innate response, vertebrates possess a second layer of protection, the
adaptive immune system
The adaptive immune system, also known as the acquired immune system, is a subsystem of the immune system that is composed of specialized, systemic cells and processes that eliminate pathogens or prevent their growth. The acquired immune system ...
, which is activated by the innate response. Here, the immune system adapts its response during an infection to improve its recognition of the pathogen. This improved response is then retained after the pathogen has been eliminated, in the form of an
immunological memory
Immunological memory is the ability of the immune system to quickly and specifically recognize an antigen that the body has previously encountered and initiate a corresponding immune response. Generally, these are secondary, tertiary and other subs ...
, and allows the adaptive immune system to mount faster and stronger attacks each time this pathogen is encountered.
Both innate and adaptive immunity depend on the ability of the immune system to distinguish between self and non-self
molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
s. In immunology, ''self'' molecules are components of an organism's body that can be distinguished from foreign substances by the immune system. Conversely, ''non-self'' molecules are those recognized as foreign molecules. One class of non-self molecules are called antigens (originally named for being ''anti''body ''gen''erators) and are defined as substances that bind to specific
immune receptor
An immune receptor (or immunologic receptor) is a receptor, usually on a cell membrane, which binds to a substance (for example, a cytokine) and causes a response in the immune system.
Types
The main receptors in the immune system are pattern r ...
s and elicit an immune response.
Surface barriers
Several barriers protect organisms from infection, including mechanical, chemical, and biological barriers. The waxy
cuticle of most leaves, the
exoskeleton
An exoskeleton (from Greek ''éxō'' "outer" and ''skeletós'' "skeleton") is an external skeleton that supports and protects an animal's body, in contrast to an internal skeleton (endoskeleton) in for example, a human. In usage, some of the ...
of insects, the
shells and membranes of externally deposited eggs, and
skin
Skin is the layer of usually soft, flexible outer tissue covering the body of a vertebrate animal, with three main functions: protection, regulation, and sensation.
Other animal coverings, such as the arthropod exoskeleton, have different de ...
are examples of mechanical barriers that are the first line of defense against infection. Organisms cannot be completely sealed from their environments, so systems act to protect body openings such as the
lungs,
intestines, and the
genitourinary tract
The genitourinary system, or urogenital system, are the organs of the reproductive system and the urinary system. These are grouped together because of their proximity to each other, their common embryological origin and the use of common pathw ...
. In the lungs, coughing and sneezing mechanically eject pathogens and other
irritants from the
respiratory tract. The flushing action of
tears
Tears are a clear liquid secreted by the lacrimal glands (tear gland) found in the eyes of all land mammals. Tears are made up of water, electrolytes, proteins, lipids, and mucins that form layers on the surface of eyes. The different types of ...
and
urine
Urine is a liquid by-product of metabolism in humans and in many other animals. Urine flows from the kidneys through the ureters to the urinary bladder. Urination results in urine being excreted from the body through the urethra.
Cellular ...
also mechanically expels pathogens, while
mucus
Mucus ( ) is a slippery aqueous secretion produced by, and covering, mucous membranes. It is typically produced from cells found in mucous glands, although it may also originate from mixed glands, which contain both serous and mucous cells. It ...
secreted by the respiratory and
gastrointestinal tract serves to trap and entangle
microorganism
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in olde ...
s.
Chemical barriers also protect against infection. The skin and respiratory tract secrete
antimicrobial peptides such as the β-
defensins.
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
s such as
lysozyme
Lysozyme (EC 3.2.1.17, muramidase, ''N''-acetylmuramide glycanhydrolase; systematic name peptidoglycan ''N''-acetylmuramoylhydrolase) is an antimicrobial enzyme produced by animals that forms part of the innate immune system. It is a glycoside ...
and
phospholipase A2 in
saliva, tears, and
breast milk are also
antibacterials
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of ...
.
Vagina
In mammals, the vagina is the elastic, muscular part of the female genital tract. In humans, it extends from the vestibule to the cervix. The outer vaginal opening is normally partly covered by a thin layer of mucosal tissue called the hymen ...
l secretions serve as a chemical barrier following
menarche, when they become slightly
acidic, while
semen
Semen, also known as seminal fluid, is an organic bodily fluid created to contain spermatozoa. It is secreted by the gonads (sexual glands) and other sexual organs of male or hermaphroditic animals and can fertilize the female ovum. Sem ...
contains defensins and
zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
to kill pathogens. In the
stomach
The stomach is a muscular, hollow organ in the gastrointestinal tract of humans and many other animals, including several invertebrates. The stomach has a dilated structure and functions as a vital organ in the digestive system. The stomach i ...
,
gastric acid serves as a chemical defense against ingested pathogens.
Within the genitourinary and gastrointestinal tracts,
commensal
Commensalism is a long-term biological interaction (symbiosis) in which members of one species gain benefits while those of the other species neither benefit nor are harmed. This is in contrast with mutualism, in which both organisms benefit fro ...
flora
Flora is all the plant life present in a particular region or time, generally the naturally occurring (indigenous (ecology), indigenous) native plant, native plants. Sometimes bacteria and fungi are also referred to as flora, as in the terms '' ...
serve as biological barriers by competing with pathogenic bacteria for food and space and, in some cases, changing the conditions in their environment, such as
pH or available iron. As a result, the probability that pathogens will reach sufficient numbers to cause illness is reduced.
Innate immune system
Microorganisms or toxins that successfully enter an organism encounter the cells and mechanisms of the innate immune system. The innate response is usually triggered when microbes are identified by
pattern recognition receptors
Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors, which detect molecules typical for the pathogens. They are proteins expressed, mainly, by cells of ...
, which recognize components that are conserved among broad groups of microorganisms,
or when damaged, injured or stressed cells send out alarm signals, many of which are recognized by the same receptors as those that recognize pathogens.
Innate immune defenses are non-specific, meaning these systems respond to pathogens in a generic way. This system does not confer long-lasting
immunity
Immunity may refer to:
Medicine
* Immunity (medical), resistance of an organism to infection or disease
* ''Immunity'' (journal), a scientific journal published by Cell Press
Biology
* Immune system
Engineering
* Radiofrequence immunity desc ...
against a pathogen. The innate immune system is the dominant system of host defense in most organisms,
and the only one in plants.
Immune sensing
Cells in the innate immune system use
pattern recognition receptor
Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors, which detect molecules typical for the pathogens. They are proteins expressed, mainly, by cells of ...
s to recognize molecular structures that are produced by pathogens.
They are
protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s expressed, mainly, by cells of the
innate immune system, such as dendritic cells, macrophages, monocytes, neutrophils and epithelial cells to identify two classes of molecules:
pathogen-associated molecular patterns
Pathogen-associated molecular patterns (PAMPs) are small molecular motifs conserved within a class of microbes. They are recognized by toll-like receptors (TLRs) and other pattern recognition receptors (PRRs) in both plants and animals. A vast arra ...
(PAMPs), which are associated with microbial
pathogens
In biology, a pathogen ( el, πάθος, "suffering", "passion" and , "producer of") in the oldest and broadest sense, is any organism or agent that can produce disease. A pathogen may also be referred to as an infectious agent, or simply a ger ...
, and
damage-associated molecular patterns
Damage-associated molecular patterns (DAMPs) are molecules within cells that are a component of the innate immune response released from damaged or dying cells due to trauma or an infection by a pathogen. They are also known as danger-associated ...
(DAMPs), which are associated with components of host's cells that are released during cell damage or cell death.
Recognition of extracellular or endosomal PAMPs is mediated by
transmembrane protein
A transmembrane protein (TP) is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequent ...
s known as
toll-like receptor
Toll-like receptors (TLRs) are a class of proteins that play a key role in the innate immune system. They are single-pass membrane-spanning receptors usually expressed on sentinel cells such as macrophages and dendritic cells, that recognize ...
s (TLRs).
TLRs share a typical structural motif, the
leucine rich repeats (LRR), which give them a curved shape. Toll-like receptors were first discovered in ''
Drosophila
''Drosophila'' () is a genus of flies, belonging to the family Drosophilidae, whose members are often called "small fruit flies" or (less frequently) pomace flies, vinegar flies, or wine flies, a reference to the characteristic of many speci ...
'' and trigger the synthesis and secretion of
cytokine
Cytokines are a broad and loose category of small proteins (~5–25 kDa) important in cell signaling. Cytokines are peptides and cannot cross the lipid bilayer of cells to enter the cytoplasm. Cytokines have been shown to be involved in autocrin ...
s and activation of other host defense programs that are necessary for both innate or adaptive immune responses. Ten toll-like receptors have been described in humans.
Cells in the innate immune system have pattern recognition receptors, which detect infection or cell damage, inside. Three major classes of these "cytosolic" receptors are
NOD–like receptors,
RIG (retinoic acid-inducible gene)-like receptors, and cytosolic DNA sensors.
Innate immune cells

Some
leukocytes
White blood cells, also called leukocytes or leucocytes, are the cells of the immune system that are involved in protecting the body against both infectious disease and foreign invaders. All white blood cells are produced and derived from mult ...
(white blood cells) act like independent, single-celled organisms and are the second arm of the innate immune system. The innate leukocytes include the
"professional" phagocytes (
macrophages,
neutrophil
Neutrophils (also known as neutrocytes or heterophils) are the most abundant type of granulocytes and make up 40% to 70% of all white blood cells in humans. They form an essential part of the innate immune system, with their functions varying ...
s, and
dendritic cells). These cells identify and eliminate pathogens, either by attacking larger pathogens through contact or by engulfing and then killing microorganisms. The other cells involved in the innate response include
innate lymphoid cell
Innate lymphoid cells (ILCs) are the most recently discovered family of innate immune cells, derived from common lymphoid progenitors (CLPs). In response to pathogenic tissue damage, ILCs contribute to immunity via the secretion of signalling mo ...
s,
mast cell
A mast cell (also known as a mastocyte or a labrocyte) is a resident cell of connective tissue that contains many granules rich in histamine and heparin. Specifically, it is a type of granulocyte derived from the myeloid stem cell that is a par ...
s,
eosinophils
Eosinophils, sometimes called eosinophiles or, less commonly, acidophils, are a variety of white blood cells (WBCs) and one of the immune system components responsible for combating multicellular parasites and certain infections in vertebrates. A ...
,
basophils
Basophils are a type of white blood cell. Basophils are the least common type of granulocyte, representing about 0.5% to 1% of circulating white blood cells. However, they are the largest type of granulocyte. They are responsible for inflammator ...
, and
natural killer cells.
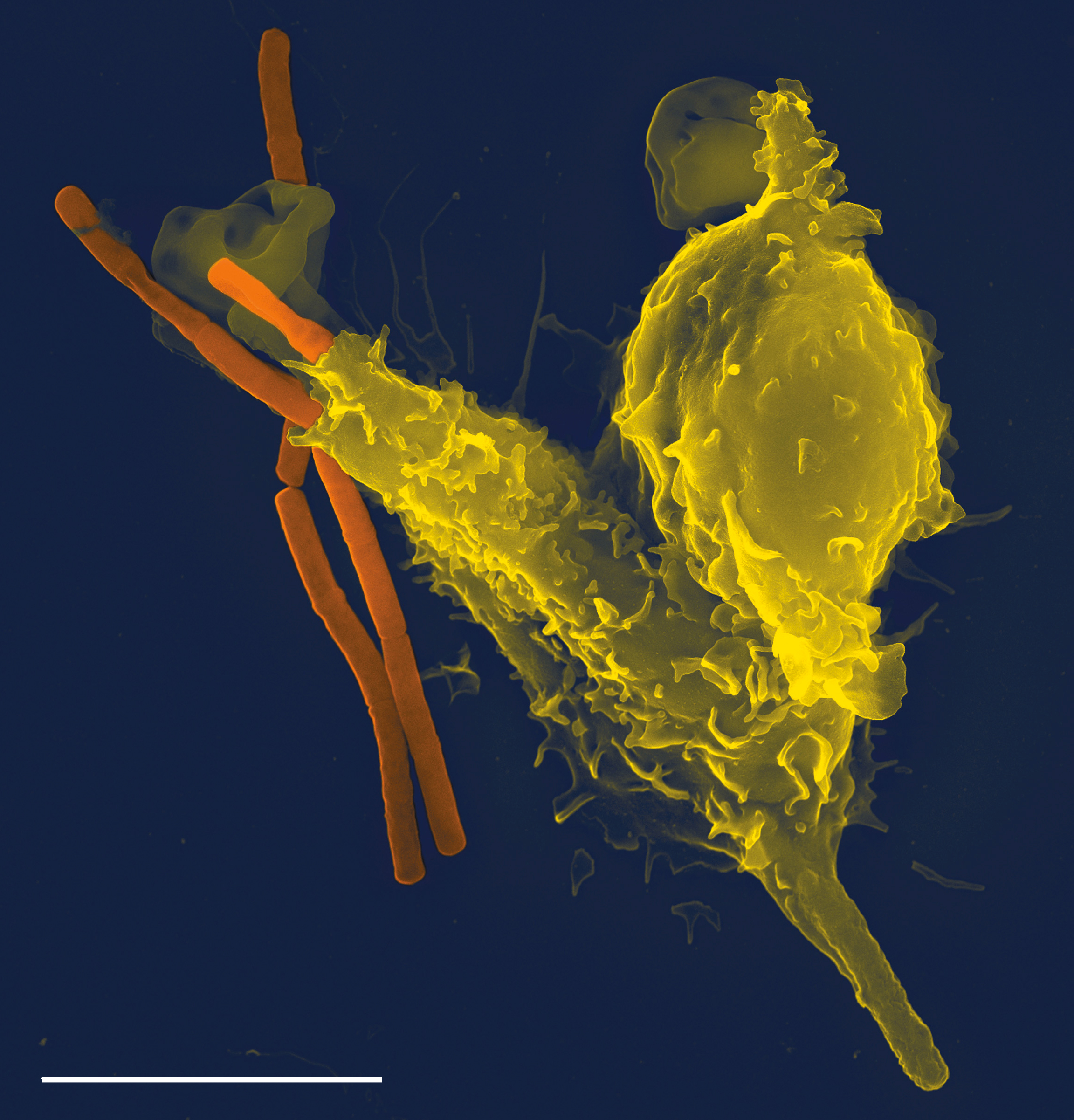
Phagocytosis
Phagocytosis () is the process by which a cell uses its plasma membrane to engulf a large particle (≥ 0.5 μm), giving rise to an internal compartment called the phagosome. It is one type of endocytosis. A cell that performs phagocytosis is ...
is an important feature of cellular innate immunity performed by cells called phagocytes that engulf pathogens or particles. Phagocytes generally patrol the body searching for pathogens, but can be called to specific locations by cytokines. Once a pathogen has been engulfed by a phagocyte, it becomes trapped in an intracellular
vesicle
Vesicle may refer to:
; In cellular biology or chemistry
* Vesicle (biology and chemistry)
In cell biology, a vesicle is a structure within or outside a cell, consisting of liquid or cytoplasm enclosed by a lipid bilayer. Vesicles form nat ...
called a
phagosome
In cell biology, a phagosome is a vesicle formed around a particle engulfed by a phagocyte via phagocytosis. Professional phagocytes include macrophages, neutrophils, and dendritic cells (DCs).
A phagosome is formed by the fusion of the cell ...
, which subsequently fuses with another vesicle called a
lysosome
A lysosome () is a membrane-bound organelle found in many animal cells. They are spherical vesicles that contain hydrolytic enzymes that can break down many kinds of biomolecules. A lysosome has a specific composition, of both its membrane pr ...
to form a
phagolysosome
In biology, a phagolysosome, or endolysosome, is a cytoplasmic body formed by the fusion of a phagosome with a lysosome in a process that occurs during phagocytosis. Formation of phagolysosomes is essential for the intracellular destruction of mic ...
. The pathogen is killed by the activity of digestive enzymes or following a
respiratory burst
Respiratory burst (or oxidative burst) is the rapid release of the reactive oxygen species (ROS), superoxide anion () and hydrogen peroxide (), from different cell types.
This is usually utilised for mammalian immunological defence, but also play ...
that releases
free radicals into the phagolysosome. Phagocytosis evolved as a means of acquiring
nutrients, but this role was extended in phagocytes to include engulfment of pathogens as a defense mechanism. Phagocytosis probably represents the oldest form of host defense, as phagocytes have been identified in both vertebrate and invertebrate animals.
Neutrophils and macrophages are phagocytes that travel throughout the body in pursuit of invading pathogens. Neutrophils are normally found in the
bloodstream
The blood circulatory system is a system of organs that includes the heart, blood vessels, and blood which is circulated throughout the entire body of a human or other vertebrate. It includes the cardiovascular system, or vascular system, tha ...
and are the most abundant type of phagocyte, representing 50% to 60% of total circulating leukocytes. During the acute phase of
inflammation
Inflammation (from la, inflammatio) is part of the complex biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or irritants, and is a protective response involving immune cells, blood vessels, and molec ...
, neutrophils migrate toward the site of inflammation in a process called
chemotaxis, and are usually the first cells to arrive at the scene of infection. Macrophages are versatile cells that reside within tissues and produce an array of chemicals including enzymes,
complement proteins
The complement system, also known as complement cascade, is a part of the immune system that enhances (complements) the ability of antibodies and phagocytic cells to clear microbes and damaged cells from an organism, promote inflammation, and a ...
, and cytokines, while they can also act as scavengers that rid the body of worn-out cells and other debris, and as
antigen-presenting cells (APC) that activate the adaptive immune system.
Dendritic cells are phagocytes in tissues that are in contact with the external environment; therefore, they are located mainly in the
skin
Skin is the layer of usually soft, flexible outer tissue covering the body of a vertebrate animal, with three main functions: protection, regulation, and sensation.
Other animal coverings, such as the arthropod exoskeleton, have different de ...
,
nose
A nose is a protuberance in vertebrates that houses the nostrils, or nares, which receive and expel air for respiration alongside the mouth. Behind the nose are the olfactory mucosa and the sinuses. Behind the nasal cavity, air next passes ...
, lungs, stomach, and intestines.
They are named for their resemblance to
neuron
A neuron, neurone, or nerve cell is an electrically excitable cell that communicates with other cells via specialized connections called synapses. The neuron is the main component of nervous tissue in all animals except sponges and placozoa. N ...
al
dendrite
Dendrites (from Greek δένδρον ''déndron'', "tree"), also dendrons, are branched protoplasmic extensions of a nerve cell that propagate the electrochemical stimulation received from other neural cells to the cell body, or soma, of the ...
s, as both have many spine-like projections. Dendritic cells serve as a link between the bodily tissues and the innate and adaptive immune systems, as they
present antigens to
T cell
A T cell is a type of lymphocyte. T cells are one of the important white blood cells of the immune system and play a central role in the adaptive immune response. T cells can be distinguished from other lymphocytes by the presence of a T-cell r ...
s, one of the key cell types of the adaptive immune system.
s are leukocytes that have granules in their cytoplasm. In this category are neutrophils, mast cells, basophils, and eosinophils. Mast cells reside in
connective tissues and
mucous membrane
A mucous membrane or mucosa is a membrane that lines various cavities in the body of an organism and covers the surface of internal organs. It consists of one or more layers of epithelial cells overlying a layer of loose connective tissue. It i ...
s, and regulate the inflammatory response. They are most often associated with
allergy
Allergies, also known as allergic diseases, refer a number of conditions caused by the hypersensitivity of the immune system to typically harmless substances in the environment. These diseases include hay fever, food allergies, atopic derm ...
and
anaphylaxis
Anaphylaxis is a serious, potentially fatal allergic reaction and medical emergency that is rapid in onset and requires immediate medical attention regardless of use of emergency medication on site. It typically causes more than one of the foll ...
.
Basophil
Basophils are a type of white blood cell. Basophils are the least common type of granulocyte, representing about 0.5% to 1% of circulating white blood cells. However, they are the largest type of granulocyte. They are responsible for inflammator ...
s and
eosinophil
Eosinophils, sometimes called eosinophiles or, less commonly, acidophils, are a variety of white blood cells (WBCs) and one of the immune system components responsible for combating multicellular parasites and certain infections in vertebrates. A ...
s are related to neutrophils. They secrete chemical mediators that are involved in defending against
parasites
Parasitism is a close relationship between species, where one organism, the parasite, lives on or inside another organism, the host, causing it some harm, and is adapted structurally to this way of life. The entomologist E. O. Wilson ha ...
and play a role in allergic reactions, such as
asthma
Asthma is a long-term inflammatory disease of the airways of the lungs. It is characterized by variable and recurring symptoms, reversible airflow obstruction, and easily triggered bronchospasms. Symptoms include episodes of wheezing, co ...
.
Innate lymphoid cells (ILCs) are a group of
innate immune cells that are derived from
common lymphoid progenitor
Lymphopoiesis (lĭm'fō-poi-ē'sĭs) (or lymphocytopoiesis) is the generation of lymphocytes, which are one of the five types of white blood cells (WBCs). It is more formally known as lymphoid hematopoiesis.
Disruption in lymphopoiesis can lead t ...
and belong to the
lymphoid lineage. These cells are defined by absence of antigen specific
B or
T cell receptor
The T-cell receptor (TCR) is a protein complex found on the surface of T cells, or T lymphocytes, that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. The binding ...
(TCR) because of the lack of
recombination activating gene
The recombination-activating genes (RAGs) encode parts of a protein complex that plays important roles in the rearrangement and recombination of the genes encoding immunoglobulin and T cell receptor molecules. There are two recombination-activa ...
. ILCs do not express myeloid or dendritic cell markers.
Natural killer cells
Natural killer cells, also known as NK cells or large granular lymphocytes (LGL), are a type of cytotoxic lymphocyte critical to the innate immune system that belong to the rapidly expanding family of known innate lymphoid cells (ILC) and represen ...
(NK) are lymphocytes and a component of the innate immune system which does not directly attack invading microbes.
Rather, NK cells destroy compromised host cells, such as tumor cells or virus-infected cells, recognizing such cells by a condition known as "missing self." This term describes cells with low levels of a cell-surface marker called MHC I (
major histocompatibility complex)—a situation that can arise in viral infections of host cells. Normal body cells are not recognized and attacked by NK cells because they express intact self MHC antigens. Those MHC antigens are recognized by killer cell immunoglobulin receptors which essentially put the brakes on NK cells.
Inflammation
Inflammation is one of the first responses of the immune system to infection.
The symptoms of inflammation are redness, swelling, heat, and pain, which are caused by increased blood flow into tissue. Inflammation is produced by
eicosanoid
Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, around 20 carbon units in length. Eicosanoids are a s ...
s and
cytokine
Cytokines are a broad and loose category of small proteins (~5–25 kDa) important in cell signaling. Cytokines are peptides and cannot cross the lipid bilayer of cells to enter the cytoplasm. Cytokines have been shown to be involved in autocrin ...
s, which are released by injured or infected cells. Eicosanoids include
prostaglandins that produce
fever
Fever, also referred to as pyrexia, is defined as having a temperature above the normal range due to an increase in the body's temperature set point. There is not a single agreed-upon upper limit for normal temperature with sources using val ...
and the
dilation
Dilation (or dilatation) may refer to:
Physiology or medicine
* Cervical dilation, the widening of the cervix in childbirth, miscarriage etc.
* Coronary dilation, or coronary reflex
* Dilation and curettage, the opening of the cervix and surgic ...
of
blood vessel
The blood vessels are the components of the circulatory system that transport blood throughout the human body. These vessels transport blood cells, nutrients, and oxygen to the tissues of the body. They also take waste and carbon dioxide away ...
s associated with inflammation, and
leukotriene
Leukotrienes are a family of eicosanoid inflammatory mediators produced in leukocytes by the oxidation of arachidonic acid (AA) and the essential fatty acid eicosapentaenoic acid (EPA) by the enzyme arachidonate 5-lipoxygenase.
Leukotrienes ...
s that attract certain
white blood cells (leukocytes).
Common cytokines include
interleukin
Interleukins (ILs) are a group of cytokines (secreted proteins and signal molecules) that are expressed and secreted by white blood cells (leukocytes) as well as some other body cells. The human genome encodes more than 50 interleukins and related ...
s that are responsible for communication between white blood cells;
chemokine
Chemokines (), or chemotactic cytokines, are a family of small cytokines or signaling proteins secreted by cells that induce directional movement of leukocytes, as well as other cell types, including endothelial and epithelial cells. In additio ...
s that promote
chemotaxis; and
interferons that have
anti-viral
Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Unlike most antibiotics, antiviral drugs do ...
effects, such as shutting down
protein synthesis in the host cell.
Growth factor
A growth factor is a naturally occurring substance capable of stimulating cell proliferation, wound healing, and occasionally cellular differentiation. Usually it is a secreted protein or a steroid hormone. Growth factors are important for regul ...
s and cytotoxic factors may also be released. These cytokines and other chemicals recruit immune cells to the site of infection and promote healing of any damaged tissue following the removal of pathogens.
The pattern-recognition receptors called
inflammasome
Inflammasomes are cytosolic multiprotein oligomers of the innate immune system responsible for the activation of inflammatory responses. Activation and assembly of the inflammasome promotes proteolytic cleavage, maturation and secretion of pro-in ...
s are multiprotein complexes (consisting of an NLR, the adaptor protein ASC, and the effector molecule pro-caspase-1) that form in response to cytosolic PAMPs and DAMPs, whose function is to generate active forms of the inflammatory cytokines IL-1β and IL-18.
Humoral defenses
The complement system is a
biochemical cascade
A biochemical cascade, also known as a signaling cascade or signaling pathway, is a series of chemical reactions that occur within a biological cell when initiated by a stimulus. This stimulus, known as a first messenger, acts on a receptor that ...
that attacks the surfaces of foreign cells. It contains over 20 different proteins and is named for its ability to "complement" the killing of pathogens by
antibodies. Complement is the major humoral component of the innate immune response.
Many species have complement systems, including non-
mammals like plants, fish, and some
invertebrate
Invertebrates are a paraphyletic group of animals that neither possess nor develop a vertebral column (commonly known as a ''backbone'' or ''spine''), derived from the notochord. This is a grouping including all animals apart from the chordate ...
s. In humans, this response is activated by complement binding to antibodies that have attached to these microbes or the binding of complement proteins to
carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or m ...
s on the surfaces of
microbes. This recognition
signal
In signal processing, a signal is a function that conveys information about a phenomenon. Any quantity that can vary over space or time can be used as a signal to share messages between observers. The '' IEEE Transactions on Signal Processing' ...
triggers a rapid killing response. The speed of the response is a result of signal amplification that occurs after sequential
proteolytic
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called protease ...
activation of complement molecules, which are also proteases. After complement proteins initially bind to the microbe, they activate their protease activity, which in turn activates other complement proteases, and so on. This produces a
catalytic
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
cascade that amplifies the initial signal by controlled
positive feedback
Positive feedback (exacerbating feedback, self-reinforcing feedback) is a process that occurs in a feedback loop which exacerbates the effects of a small disturbance. That is, the effects of a perturbation on a system include an increase in th ...
. The cascade results in the production of peptides that attract immune cells, increase
vascular permeability
Vascular permeability, often in the form of capillary permeability or microvascular permeability, characterizes the capacity of a blood vessel wall to allow for the flow of small molecules (drugs, nutrients, water, ions) or even whole cells (lymph ...
, and
opsonize
Opsonins are extracellular proteins that, when bound to substances or cells, induce phagocytes to phagocytose the substances or cells with the opsonins bound. Thus, opsonins act as tags to label things in the body that should be phagocytosed (i.e. ...
(coat) the surface of a pathogen, marking it for destruction. This deposition of complement can also kill cells directly by disrupting their
plasma membrane.
Adaptive immune system

The adaptive immune system evolved in early vertebrates and allows for a stronger immune response as well as
immunological memory
Immunological memory is the ability of the immune system to quickly and specifically recognize an antigen that the body has previously encountered and initiate a corresponding immune response. Generally, these are secondary, tertiary and other subs ...
, where each pathogen is "remembered" by a signature antigen. The adaptive immune response is antigen-specific and requires the recognition of specific "non-self" antigens during a process called
antigen presentation
Antigen presentation is a vital immune process that is essential for T cell immune response triggering. Because T cells recognize only fragmented antigens displayed on cell surfaces, antigen processing must occur before the antigen fragment, n ...
. Antigen specificity allows for the generation of responses that are tailored to specific pathogens or pathogen-infected cells. The ability to mount these tailored responses is maintained in the body by "memory cells". Should a pathogen infect the body more than once, these specific memory cells are used to quickly eliminate it.
Recognition of antigen
The cells of the adaptive immune system are special types of leukocytes, called lymphocytes.
B cells and T cells are the major types of lymphocytes and are derived from
hematopoietic stem cells in the
bone marrow. B cells are involved in the
humoral immune response
Humoral immunity is the aspect of immunity that is mediated by macromolecules - including secreted antibodies, complement proteins, and certain antimicrobial peptides - located in extracellular fluids. Humoral immunity is named so because it in ...
, whereas T cells are involved in
cell-mediated immune response. Killer T cells only recognize antigens coupled to
Class I MHC molecules, while helper T cells and regulatory T cells only recognize antigens coupled to
Class II MHC
MHC Class II molecules are a class of major histocompatibility complex (MHC) molecules normally found only on professional antigen-presenting cells such as dendritic cells, mononuclear phagocytes, some endothelial cells, thymic epithelial ce ...
molecules. These two mechanisms of antigen presentation reflect the different roles of the two types of T cell. A third, minor subtype are the
γδ T cells that recognize intact antigens that are not bound to MHC receptors.
The double-positive T cells are exposed to a wide variety of
self-antigens in the
thymus
The thymus is a specialized primary lymphoid organ of the immune system. Within the thymus, thymus cell lymphocytes or ''T cells'' mature. T cells are critical to the adaptive immune system, where the body adapts to specific foreign invaders. ...
, in which
iodine is necessary for its thymus development and activity.
In contrast, the B cell antigen-specific receptor is an antibody molecule on the B cell surface and recognizes native (unprocessed) antigen without any need for
antigen processing Antigen processing, or the cytosolic pathway, is an immunological process that prepares antigens for presentation to special cells of the immune system called T lymphocytes. It is considered to be a stage of antigen presentation pathways. This pro ...
. Such antigens may be large molecules found on the surfaces of pathogens, but can also be small
hapten
In immunology, haptens are small molecules that elicit an immune response only when attached to a large carrier such as a protein; the carrier may be one that also does not elicit an immune response by itself (in general, only large molecules, i ...
s (such as penicillin) attached to carrier molecule. Each lineage of B cell expresses a different antibody, so the complete set of B cell antigen receptors represent all the antibodies that the body can manufacture. When B or T cells encounter their related antigens they multiply and many "clones" of the cells are produced that target the same antigen. This is called
clonal selection
In immunology, clonal selection theory explains the functions of cells of the immune system (lymphocytes) in response to specific antigens invading the body. The concept was introduced by Australian doctor Frank Macfarlane Burnet in 1957, in an ...
.
Antigen presentation to T lymphocytes
Both B cells and T cells carry receptor molecules that recognize specific targets. T cells recognize a "non-self" target, such as a pathogen, only after antigens (small fragments of the pathogen) have been processed and presented in combination with a "self" receptor called a major histocompatibility complex (MHC) molecule.
Cell mediated immunity
There are two major subtypes of T cells: the
killer T cell and the
helper T cell
The T helper cells (Th cells), also known as CD4+ cells or CD4-positive cells, are a type of T cell that play an important role in the adaptive immune system. They aid the activity of other immune cells by releasing cytokines. They are consider ...
. In addition there are
regulatory T cells
The regulatory T cells (Tregs or Treg cells), formerly known as suppressor T cells, are a subpopulation of T cells that modulate the immune system, maintain tolerance to self-antigens, and prevent autoimmune disease. Treg cells are immunosu ...
which have a role in modulating immune response.
Killer T cells
Killer T cells
A cytotoxic T cell (also known as TC, cytotoxic T lymphocyte, CTL, T-killer cell, cytolytic T cell, CD8+ T-cell or killer T cell) is a T lymphocyte (a type of white blood cell) that kills cancer cells, cells that are infected by intracellular pa ...
are a sub-group of T cells that kill cells that are infected with viruses (and other pathogens), or are otherwise damaged or dysfunctional. As with B cells, each type of T cell recognizes a different antigen. Killer T cells are activated when their
T-cell receptor
The T-cell receptor (TCR) is a protein complex found on the surface of T cells, or T lymphocytes, that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. The binding b ...
binds to this specific antigen in a complex with the MHC Class I receptor of another cell. Recognition of this MHC:antigen complex is aided by a
co-receptor
A co-receptor is a cell surface receptor that binds a signalling molecule in addition to a primary receptor in order to facilitate ligand recognition and initiate biological processes, such as entry of a pathogen into a host cell.
Properties
The ...
on the T cell, called
CD8
CD8 (cluster of differentiation 8) is a transmembrane glycoprotein that serves as a co-receptor for the T-cell receptor (TCR). Along with the TCR, the CD8 co-receptor plays a role in T cell signaling and aiding with cytotoxic T cell-antigen int ...
. The T cell then travels throughout the body in search of cells where the MHC I receptors bear this antigen. When an activated T cell contacts such cells, it releases
cytotoxins
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are an immune cell or some types of venom, e.g. from the puff adder (''Bitis arietans'') or brown recluse spider (''Loxosceles reclusa'').
Cell physiology
Treating cell ...
, such as
perforin
Perforin-1 is a protein that in humans is encoded by the ''PRF1'' gene and the ''Prf1'' gene in mice.
Function
Perforin is a pore forming cytolytic protein found in the granules of cytotoxic T lymphocytes (CTLs) and natural killer cells (NK cel ...
, which form pores in the target cell's
plasma membrane, allowing
ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
s, water and toxins to enter. The entry of another toxin called
granulysin (a protease) induces the target cell to undergo
apoptosis.
T cell killing of host cells is particularly important in preventing the replication of viruses. T cell activation is tightly controlled and generally requires a very strong MHC/antigen activation signal, or additional activation signals provided by "helper" T cells (see below).
Helper T cells
Helper T cells
The T helper cells (Th cells), also known as CD4+ cells or CD4-positive cells, are a type of T cell that play an important role in the adaptive immune system. They aid the activity of other immune cells by releasing cytokines. They are considere ...
regulate both the innate and adaptive immune responses and help determine which immune responses the body makes to a particular pathogen. These cells have no cytotoxic activity and do not kill infected cells or clear pathogens directly. They instead control the immune response by directing other cells to perform these tasks.
Helper T cells express T cell receptors that recognize antigen bound to Class II MHC molecules. The MHC:antigen complex is also recognized by the helper cell's
CD4 co-receptor, which recruits molecules inside the T cell (such as
Lck
Lck (or lymphocyte-specific protein tyrosine kinase) is a 56 kDa protein that is found inside specialized cells of the immune system called lymphocytes. The Lck is a member of Src kinase family (SFK), it is important for the activation of the T ...
) that are responsible for the T cell's activation. Helper T cells have a weaker association with the MHC:antigen complex than observed for killer T cells, meaning many receptors (around 200–300) on the helper T cell must be bound by an MHC:antigen to activate the helper cell, while killer T cells can be activated by engagement of a single MHC:antigen molecule. Helper T cell activation also requires longer duration of engagement with an antigen-presenting cell. The activation of a resting helper T cell causes it to release cytokines that influence the activity of many cell types. Cytokine signals produced by helper T cells enhance the microbicidal function of macrophages and the activity of killer T cells. In addition, helper T cell activation causes an upregulation of molecules expressed on the T cell's surface, such as CD40 ligand (also called
CD154
CD154, also called CD40 ligand or CD40L, is a protein that is primarily expressed on activated T cells and is a member of the TNF superfamily of molecules. It binds to CD40 on antigen-presenting cells (APC), which leads to many effects depending ...
), which provide extra stimulatory signals typically required to activate antibody-producing B cells.
Gamma delta T cells
Gamma delta T cell
Gamma delta T cells (γδ T cells) are T cells that have a γδ T-cell receptor (TCR) on their surface. Most T cells are αβ (alpha beta) T cells with TCR composed of two glycoprotein chains called α (alpha) and β (beta) TCR chains. In contrast, ...
s (γδ T cells) possess an alternative T-cell receptor (TCR) as opposed to CD4+ and CD8+ (αβ) T cells and share the characteristics of helper T cells, cytotoxic T cells and NK cells. The conditions that produce responses from γδ T cells are not fully understood. Like other 'unconventional' T cell subsets bearing invariant TCRs, such as
CD1d
CD1D is the human gene that encodes the protein CD1d, a member of the CD1 (cluster of differentiation 1) family of glycoproteins expressed on the surface of various human antigen-presenting cells. They are non-classical MHC proteins, related to ...
-restricted
natural killer T cell
Natural killer T (NKT) cells are a heterogeneous group of T cells that share properties of both T cells and natural killer cells. Many of these cells recognize the non-polymorphic CD1d molecule, an antigen-presenting molecule that binds self an ...
s, γδ T cells straddle the border between innate and adaptive immunity. On one hand, γδ T cells are a component of adaptive immunity as they
rearrange TCR genes to produce receptor diversity and can also develop a memory phenotype. On the other hand, the various subsets are also part of the innate immune system, as restricted TCR or NK receptors may be used as
pattern recognition receptor
Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors, which detect molecules typical for the pathogens. They are proteins expressed, mainly, by cells of ...
s. For example, large numbers of human Vγ9/Vδ2 T cells respond within hours to
common molecules produced by microbes, and highly restricted Vδ1+ T cells in
epithelia
Epithelium or epithelial tissue is one of the four basic types of animal tissue, along with connective tissue, muscle tissue and nervous tissue. It is a thin, continuous, protective layer of compactly packed cells with a little intercellula ...
respond to stressed epithelial cells.
Humoral immune response

A
B cell identifies pathogens when antibodies on its surface bind to a specific foreign antigen.
This antigen/antibody complex is taken up by the B cell and processed by
proteolysis into
peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
...
s. The B cell then displays these antigenic peptides on its surface MHC class II molecules. This combination of MHC and antigen attracts a matching helper T cell, which releases
lymphokine Lymphokines are a subset of cytokines that are produced by a type of immune cell known as a lymphocyte. They are protein mediators typically produced by T cells to direct the immune system response by signaling between its cells. Lymphokines have ...
s and activates the B cell. As the activated B cell then begins to
divide, its offspring (
plasma cells
Plasma cells, also called plasma B cells or effector B cells, are white blood cells that originate in the lymphoid organs as B lymphocytes and secrete large quantities of proteins called antibodies in response to being presented specific substan ...
)
secrete 440px
Secretion is the movement of material from one point to another, such as a secreted chemical substance from a cell or gland. In contrast, excretion is the removal of certain substances or waste products from a cell or organism. The classical ...
millions of copies of the antibody that recognizes this antigen. These antibodies circulate in
blood plasma
Blood plasma is a light amber-colored liquid component of blood in which blood cells are absent, but contains proteins and other constituents of whole blood in suspension. It makes up about 55% of the body's total blood volume. It is the intr ...
and
lymph
Lymph (from Latin, , meaning "water") is the fluid that flows through the lymphatic system, a system composed of lymph vessels (channels) and intervening lymph nodes whose function, like the venous system, is to return fluid from the tissues ...
, bind to pathogens expressing the antigen and mark them for destruction by
complement activation
The complement system, also known as complement cascade, is a part of the immune system that enhances (complements) the ability of antibodies and phagocytic
Phagocytosis () is the process by which a cell uses its plasma membrane to engulf a ...
or for uptake and destruction by
phagocyte
Phagocytes are cells that protect the body by ingesting harmful foreign particles, bacteria, and dead or dying cells. Their name comes from the Greek ', "to eat" or "devour", and "-cyte", the suffix in biology denoting "cell", from the Greek ...
s. Antibodies can also neutralize challenges directly, by binding to bacterial toxins or by interfering with the receptors that viruses and bacteria use to infect cells.
Newborn infants have no prior exposure to microbes and are particularly vulnerable to infection. Several layers of passive protection are provided by the mother. During pregnancy, a particular type of antibody, called
IgG
Immunoglobulin G (Ig G) is a type of antibody. Representing approximately 75% of serum antibodies in humans, IgG is the most common type of antibody found in blood circulation. IgG molecules are created and released by plasma B cells. Each IgG ...
, is transported from mother to baby directly through the
placenta
The placenta is a temporary embryonic and later fetal organ that begins developing from the blastocyst shortly after implantation. It plays critical roles in facilitating nutrient, gas and waste exchange between the physically separate mate ...
, so human babies have high levels of antibodies even at birth, with the same range of antigen specificities as their mother. Breast milk or
colostrum
Colostrum, also known as beestings or first milk, is the first form of milk produced by the mammary glands of mammals (including humans) immediately following delivery of the newborn. Colostrum powder is rich in high protein and low in sugar and ...
also contains antibodies that are transferred to the gut of the infant and protect against bacterial infections until the newborn can synthesize its own antibodies. This is
passive immunity Passive immunity is the transfer of active humoral immunity of ready-made antibodies. Passive immunity can occur naturally, when maternal antibodies are transferred to the fetus through the placenta, and it can also be induced artificially, when ...
because the
fetus
A fetus or foetus (; plural fetuses, feti, foetuses, or foeti) is the unborn offspring that develops from an animal embryo. Following embryonic development the fetal stage of development takes place. In human prenatal development, fetal dev ...
does not actually make any memory cells or antibodies—it only borrows them. This passive immunity is usually short-term, lasting from a few days up to several months. In medicine, protective passive immunity can also be
transferred artificially from one individual to another.
Immunological memory
When B cells and T cells are activated and begin to replicate, some of their offspring become long-lived memory cells. Throughout the lifetime of an animal, these memory cells remember each specific pathogen encountered and can mount a strong response if the pathogen is detected again. This is "adaptive" because it occurs during the lifetime of an individual as an adaptation to infection with that pathogen and prepares the immune system for future challenges. Immunological memory can be in the form of either passive short-term memory or active long-term memory.
Physiological regulation

The immune system is involved in many aspects of physiological regulation in the body. The immune system interacts intimately with other systems, such as the
endocrine and the
nervous systems. The immune system also plays a crucial role in
embryogenesis (development of the embryo), as well as in
tissue repair and
regeneration
Regeneration may refer to:
Science and technology
* Regeneration (biology), the ability to recreate lost or damaged cells, tissues, organs and limbs
* Regeneration (ecology), the ability of ecosystems to regenerate biomass, using photosynthesis
...
.
Hormones
Hormone
A hormone (from the Greek participle , "setting in motion") is a class of signaling molecules in multicellular organisms that are sent to distant organs by complex biological processes to regulate physiology and behavior. Hormones are require ...
s can act as
immunomodulators
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as ''activation immunotherapies,'' while immunotherap ...
, altering the sensitivity of the immune system. For example,
female sex hormones are known
immunostimulator
Immunostimulants, also known as immunostimulators, are substances (drugs and nutrients) that stimulate the immune system by inducing activation or increasing activity of any of its components. One notable example is the granulocyte macrophage colo ...
s of both adaptive and innate immune responses. Some autoimmune diseases such as
lupus erythematosus
Lupus erythematosus is a collection of autoimmune diseases in which the human immune system becomes hyperactive and attacks healthy tissues. Symptoms of these diseases can affect many different body systems, including joints, skin, kidneys, blo ...
strike women preferentially, and their onset often coincides with
puberty
Puberty is the process of physical changes through which a child's body matures into an adult body capable of sexual reproduction. It is initiated by hormonal signals from the brain to the gonads: the ovaries in a girl, the testes in a bo ...
. By contrast,
male sex hormones
An androgen (from Greek ''andr-'', the stem of the word meaning "man") is any natural or synthetic steroid hormone that regulates the development and maintenance of male characteristics in vertebrates by binding to androgen receptors. This in ...
such as
testosterone
Testosterone is the primary sex hormone and anabolic steroid in males. In humans, testosterone plays a key role in the development of male reproductive tissues such as testes and prostate, as well as promoting secondary sexual characteristi ...
seem to be
immunosuppressive
Immunosuppression is a reduction of the activation or efficacy of the immune system. Some portions of the immune system itself have immunosuppressive effects on other parts of the immune system, and immunosuppression may occur as an adverse reacti ...
. Other hormones appear to regulate the immune system as well, most notably
prolactin
Prolactin (PRL), also known as lactotropin, is a protein best known for its role in enabling mammals to produce milk. It is influential in over 300 separate processes in various vertebrates, including humans. Prolactin is secreted from the pit ...
,
growth hormone
Growth hormone (GH) or somatotropin, also known as human growth hormone (hGH or HGH) in its human form, is a peptide hormone that stimulates growth, cell reproduction, and cell regeneration in humans and other animals. It is thus important in h ...
and
vitamin D
Vitamin D is a group of fat-soluble secosteroids responsible for increasing intestinal absorption of calcium, magnesium, and phosphate, and many other biological effects. In humans, the most important compounds in this group are vitamin D3 (c ...
.
Vitamin D
Although cellular studies indicate that vitamin D has receptors and probable functions in the immune system, there is no
clinical evidence to prove that
vitamin D deficiency
Vitamin D deficiency or hypovitaminosis D is a vitamin D level that is below normal. It most commonly occurs in people when they have inadequate exposure to sunlight, particularly sunlight with adequate ultraviolet B rays (UVB). Vitamin D defic ...
increases the risk for immune diseases or vitamin D
supplementation lowers immune disease risk.
A 2011 United States
Institute of Medicine report stated that "outcomes related to ... immune functioning and
autoimmune disorder
An autoimmune disease is a condition arising from an abnormal immune response to a functioning body part. At least 80 types of autoimmune diseases have been identified, with some evidence suggesting that there may be more than 100 types. Nearly ...
s, and infections ... could not be linked reliably with calcium or vitamin D intake and were often conflicting."
Sleep and rest
The immune system is affected by sleep and rest, and
sleep deprivation is detrimental to immune function. Complex feedback loops involving
cytokines, such as
interleukin-1
The Interleukin-1 family (IL-1 family) is a group of 11 cytokines that plays a central role in the regulation of immune and inflammatory responses to infections or sterile insults.
Discovery
Discovery of these cytokines began with studies on t ...
and
tumor necrosis factor-α
Tumor necrosis factor (TNF, cachexin, or cachectin; formerly known as tumor necrosis factor alpha or TNF-α) is an adipokine and a cytokine. TNF is a member of the TNF superfamily, which consists of various transmembrane proteins with a homolog ...
produced in response to infection, appear to also play a role in the regulation of non-rapid eye movement (
REM) sleep. Thus the immune response to infection may result in changes to the sleep cycle, including an increase in
slow-wave sleep
Slow-wave sleep (SWS), often referred to as deep sleep, consists of stage three of non-rapid eye movement sleep. It usually lasts between 70 and 90 minutes and takes place during the first hours of the night. Initially, SWS consisted of both St ...
relative to REM sleep.
In people with sleep deprivation,
active immunizations may have a diminished effect and may result in lower antibody production, and a lower immune response, than would be noted in a well-rested individual.
Additionally, proteins such as
NFIL3, which have been shown to be closely intertwined with both T-cell differentiation and
circadian rhythms, can be affected through the disturbance of natural light and dark cycles through instances of sleep deprivation. These disruptions can lead to an increase in chronic conditions such as heart disease, chronic pain, and asthma.
In addition to the negative consequences of sleep deprivation, sleep and the intertwined circadian system have been shown to have strong regulatory effects on immunological functions affecting both innate and adaptive immunity. First, during the early slow-wave-sleep stage, a sudden drop in blood levels of
cortisol,
epinephrine, and
norepinephrine
Norepinephrine (NE), also called noradrenaline (NA) or noradrenalin, is an organic chemical in the catecholamine family that functions in the brain and body as both a hormone and neurotransmitter. The name "noradrenaline" (from Latin '' ad' ...
causes increased blood levels of the hormones
leptin,
pituitary growth hormone, and
prolactin
Prolactin (PRL), also known as lactotropin, is a protein best known for its role in enabling mammals to produce milk. It is influential in over 300 separate processes in various vertebrates, including humans. Prolactin is secreted from the pit ...
. These signals induce a pro-inflammatory state through the production of the pro-inflammatory cytokines interleukin-1,
interleukin-12
Interleukin 12 (IL-12) is an interleukin that is naturally produced by dendritic cells, macrophages, neutrophils, and human B- lymphoblastoid cells ( NC-37) in response to antigenic stimulation. IL-12 belongs to the family of interleukin-12. ...
,
TNF-alpha
Tumor necrosis factor (TNF, cachexin, or cachectin; formerly known as tumor necrosis factor alpha or TNF-α) is an adipokine and a cytokine. TNF is a member of the TNF superfamily, which consists of various transmembrane proteins with a homolog ...
and
IFN-gamma
Interferon gamma (IFN-γ) is a dimerized soluble cytokine that is the only member of the type II class of interferons. The existence of this interferon, which early in its history was known as immune interferon, was described by E. F. Wheelock ...
. These cytokines then stimulate immune functions such as immune cell activation, proliferation, and
differentiation. During this time of a slowly evolving adaptive immune response, there is a peak in undifferentiated or less differentiated cells, like naïve and central memory T cells. In addition to these effects, the milieu of hormones produced at this time (leptin, pituitary growth hormone, and prolactin) supports the interactions between APCs and T-cells, a shift of the
Th1/Th2 cytokine balance towards one that supports T
h1, an increase in overall T
h cell proliferation, and naïve T cell migration to lymph nodes. This is also thought to support the formation of long-lasting immune memory through the initiation of Th1 immune responses.
During wake periods, differentiated effector cells, such as cytotoxic natural killer cells and cytotoxic T lymphocytes, peak to elicit an effective response against any intruding pathogens. Anti-inflammatory molecules, such as cortisol and
catecholamine
A catecholamine (; abbreviated CA) is a monoamine neurotransmitter, an organic compound that has a catechol (benzene with two hydroxyl side groups next to each other) and a side-chain amine.
Catechol can be either a free molecule or a su ...
s, also peak during awake active times. Inflammation would cause serious cognitive and physical impairments if it were to occur during wake times, and inflammation may occur during sleep times due to the presence of
melatonin. Inflammation causes a great deal of
oxidative stress
Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage. Disturbances in the normal ...
and the presence of melatonin during sleep times could actively counteract free radical production during this time.
Physical exercise
Physical exercise has a positive effect on the immune system and depending on the frequency and intensity, the pathogenic effects of diseases caused by bacteria and viruses are moderated.
Immediately after intense exercise there is a transient immunodepression, where the number of circulating lymphocytes decreases and antibody production declines. This may give rise to a window of opportunity for infection and reactivation of latent virus infections,
but the evidence is inconclusive.
Changes at the cellular level

During exercise there is an increase in circulating
white blood cells
White blood cells, also called leukocytes or leucocytes, are the cells of the immune system that are involved in protecting the body against both infectious disease and foreign invaders. All white blood cells are produced and derived from mult ...
of all types. This is caused by the frictional force of blood flowing on the
endothelial cell surface and
catecholamine
A catecholamine (; abbreviated CA) is a monoamine neurotransmitter, an organic compound that has a catechol (benzene with two hydroxyl side groups next to each other) and a side-chain amine.
Catechol can be either a free molecule or a su ...
s affecting
β-adrenergic receptor
The adrenergic receptors or adrenoceptors are a class of G protein-coupled receptors that are targets of many catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) produced by the body, but also many medications like beta ...
s (βARs).
The number of
neutrophils in the blood increases and remains raised for up to six hours and
immature forms are present. Although the increase in neutrophils ("
neutrophilia
Neutrophilia (also called neutrophil leukocytosis or occasionally neutrocytosis) is leukocytosis of neutrophils, that is, a high number of neutrophils in the blood. Because neutrophils are the main type of granulocytes, mentions of granulocytosi ...
") is similar to that seen during bacterial infections, after exercise the cell population returns to normal by around 24 hours.
The number of circulating
lymphocyte
A lymphocyte is a type of white blood cell (leukocyte) in the immune system of most vertebrates. Lymphocytes include natural killer cells (which function in cell-mediated, cytotoxic innate immunity), T cells (for cell-mediated, cytotoxic ad ...
s (mainly
natural killer cells) decreases during intense exercise but returns to normal after 4 to 6 hours. Although up to 2% of the cells
die most migrate from the blood to the tissues, mainly the intestines and lungs, where
pathogen
In biology, a pathogen ( el, πάθος, "suffering", "passion" and , "producer of") in the oldest and broadest sense, is any organism or agent that can produce disease. A pathogen may also be referred to as an infectious agent, or simply a germ ...
s are most likely to be encountered.
Some
monocyte
Monocytes are a type of leukocyte or white blood cell. They are the largest type of leukocyte in blood and can differentiate into macrophages and conventional dendritic cells. As a part of the vertebrate innate immune system monocytes also ...
s leave the blood circulation and migrate to the muscles where they differentiate and become
macrophages.
These cells differentiate into two types: proliferative macrophages, which are responsible for increasing the number of
stem cells and restorative macrophages, which are involved their maturing to muscle cells.
Repair and regeneration
The immune system, particularly the innate component, plays a decisive role in tissue repair after an
insult. Key actors include
macrophages and
neutrophil
Neutrophils (also known as neutrocytes or heterophils) are the most abundant type of granulocytes and make up 40% to 70% of all white blood cells in humans. They form an essential part of the innate immune system, with their functions varying ...
s, but other cellular actors, including γδ T cells,
innate lymphoid cell
Innate lymphoid cells (ILCs) are the most recently discovered family of innate immune cells, derived from common lymphoid progenitors (CLPs). In response to pathogenic tissue damage, ILCs contribute to immunity via the secretion of signalling mo ...
s (ILCs), and
regulatory T cell
The regulatory T cells (Tregs or Treg cells), formerly known as suppressor T cells, are a subpopulation of T cells that modulate the immune system, maintain tolerance to self-antigens, and prevent autoimmune disease. Treg cells are immunosu ...
s (Tregs), are also important. The plasticity of immune cells and the balance between pro-inflammatory and anti-inflammatory signals are crucial aspects of efficient tissue repair. Immune components and pathways are involved in regeneration as well, for example in
amphibians such as in
axolotl limb regeneration. According to one hypothesis, organisms that can regenerate (''e.g.'',
axolotl
The axolotl (; from nci, āxōlōtl ), ''Ambystoma mexicanum'', is a paedomorphic salamander closely related to the tiger salamander. Axolotls are unusual among amphibians in that they reach adulthood without undergoing metamorphosis. I ...
s) could be less immunocompetent than organisms that cannot regenerate.
Disorders of human immunity
Failures of host defense occur and fall into three broad categories: immunodeficiencies, autoimmunity, and hypersensitivities.
Immunodeficiencies
Immunodeficiencies occur when one or more of the components of the immune system are inactive. The ability of the immune system to respond to pathogens is diminished in both the young and the
elderly, with immune responses beginning to decline at around 50 years of age due to
immunosenescence
Immunosenescence is the gradual deterioration of the immune system, brought on by natural age advancement. A 2020 review concluded that the adaptive immune system is affected more than the innate immune system. Immunosenescence involves both the ...
.
In
developed countries
A developed country (or industrialized country, high-income country, more economically developed country (MEDC), advanced country) is a sovereign state that has a high quality of life, developed economy and advanced technological infrastruct ...
,
obesity
Obesity is a medical condition, sometimes considered a disease, in which excess body fat has accumulated to such an extent that it may negatively affect health. People are classified as obese when their body mass index (BMI)—a person's ...
,
alcoholism
Alcoholism is, broadly, any drinking of alcohol that results in significant mental or physical health problems. Because there is disagreement on the definition of the word ''alcoholism'', it is not a recognized diagnostic entity. Predomi ...
, and drug use are common causes of poor immune function, while
malnutrition
Malnutrition occurs when an organism gets too few or too many nutrients, resulting in health problems. Specifically, it is "a deficiency, excess, or imbalance of energy, protein and other nutrients" which adversely affects the body's tissues ...
is the most common cause of immunodeficiency in
developing countries
A developing country is a sovereign state with a lesser developed industrial base and a lower Human Development Index (HDI) relative to other countries. However, this definition is not universally agreed upon. There is also no clear agreem ...
.
Diets lacking sufficient protein are associated with impaired cell-mediated immunity, complement activity, phagocyte function,
IgA antibody concentrations, and cytokine production. Additionally, the loss of the
thymus
The thymus is a specialized primary lymphoid organ of the immune system. Within the thymus, thymus cell lymphocytes or ''T cells'' mature. T cells are critical to the adaptive immune system, where the body adapts to specific foreign invaders. ...
at an early age through
genetic mutation
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, ...
or surgical removal results in severe immunodeficiency and a high susceptibility to infection. Immunodeficiencies can also be inherited or '
acquired'.
Severe combined immunodeficiency
Severe combined immunodeficiency (SCID), also known as Swiss-type agammaglobulinemia, is a rare genetic disorder characterized by the disturbed development of functional T cells and B cells caused by numerous genetic mutations that result in diffe ...
is a rare
genetic disorder
A genetic disorder is a health problem caused by one or more abnormalities in the genome. It can be caused by a mutation in a single gene (monogenic) or multiple genes (polygenic) or by a chromosomal abnormality. Although polygenic disorders ...
characterized by the disturbed development of functional T cells and B cells caused by numerous genetic mutations.
Chronic granulomatous disease
Chronic granulomatous disease (CGD), also known as Bridges–Good syndrome, chronic granulomatous disorder, and Quie syndrome, is a diverse group of hereditary diseases in which certain cells of the immune system have difficulty forming the reacti ...
, where
phagocyte
Phagocytes are cells that protect the body by ingesting harmful foreign particles, bacteria, and dead or dying cells. Their name comes from the Greek ', "to eat" or "devour", and "-cyte", the suffix in biology denoting "cell", from the Greek ...
s have a reduced ability to destroy pathogens, is an example of an inherited, or
congenital, immunodeficiency.
AIDS and some types of
cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
cause acquired immunodeficiency.
Autoimmunity
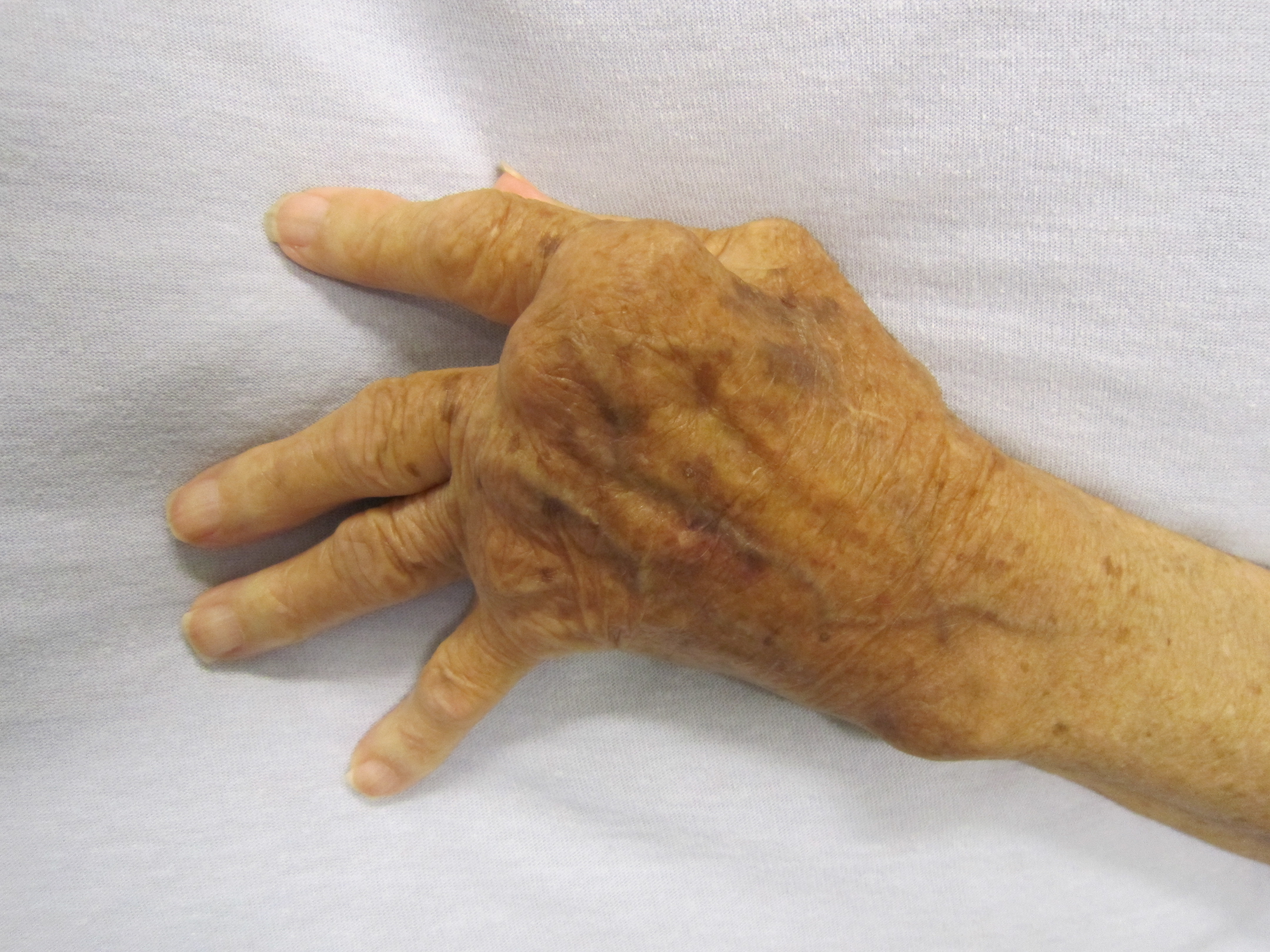
Overactive immune responses form the other end of immune dysfunction, particularly the
autoimmune disorders
An autoimmune disease is a condition arising from an abnormal immune response to a functioning body part. At least 80 types of autoimmune diseases have been identified, with some evidence suggesting that there may be more than 100 types. Nearly a ...
. Here, the immune system fails to properly distinguish between
self
The self is an individual as the object of that individual’s own reflective consciousness. Since the ''self'' is a reference by a subject to the same subject, this reference is necessarily subjective. The sense of having a self—or ''selfhoo ...
and non-self, and attacks part of the body. Under normal circumstances, many T cells and antibodies react with "self" peptides. One of the functions of specialized cells (located in the
thymus
The thymus is a specialized primary lymphoid organ of the immune system. Within the thymus, thymus cell lymphocytes or ''T cells'' mature. T cells are critical to the adaptive immune system, where the body adapts to specific foreign invaders. ...
and bone marrow) is to present young lymphocytes with
self antigens produced throughout the body and to eliminate those cells that recognize
self-antigens, preventing autoimmunity.
Common autoimmune diseases include
Hashimoto's thyroiditis
Hashimoto's thyroiditis, also known as chronic lymphocytic thyroiditis and Hashimoto's disease, is an autoimmune disease in which the thyroid gland is gradually destroyed. Early on, symptoms may not be noticed. Over time, the thyroid may enlarg ...
,
rheumatoid arthritis
Rheumatoid arthritis (RA) is a long-term autoimmune disorder that primarily affects joints. It typically results in warm, swollen, and painful joints. Pain and stiffness often worsen following rest. Most commonly, the wrist and hands are invol ...
,
diabetes mellitus type 1
Type 1 diabetes (T1D), formerly known as juvenile diabetes, is an autoimmune disease that originates when cells that make insulin (beta cells) are destroyed by the immune system. Insulin is a hormone required for the cells to use blood sugar for ...
,
and
systemic lupus erythematosus.
Hypersensitivity
Hypersensitivity
Hypersensitivity (also called hypersensitivity reaction or intolerance) refers to undesirable reactions produced by the normal immune system, including allergies and autoimmunity. They are usually referred to as an over-reaction of the immune ...
is an immune response that damages the body's own tissues. It is divided into four classes (Type I – IV) based on the mechanisms involved and the time course of the hypersensitive reaction. Type I hypersensitivity is an immediate or
anaphylactic
Anaphylaxis is a serious, potentially fatal allergic reaction and medical emergency that is rapid in onset and requires immediate medical attention regardless of use of emergency medication on site. It typically causes more than one of the follo ...
reaction, often associated with allergy. Symptoms can range from mild discomfort to death. Type I hypersensitivity is mediated by
IgE
Immunoglobulin E (IgE) is a type of antibody (or immunoglobulin (Ig) " isotype") that has been found only in mammals. IgE is synthesised by plasma cells. Monomers of IgE consist of two heavy chains (ε chain) and two light chains, with the ε c ...
, which triggers degranulation of
mast cell
A mast cell (also known as a mastocyte or a labrocyte) is a resident cell of connective tissue that contains many granules rich in histamine and heparin. Specifically, it is a type of granulocyte derived from the myeloid stem cell that is a par ...
s and
basophils
Basophils are a type of white blood cell. Basophils are the least common type of granulocyte, representing about 0.5% to 1% of circulating white blood cells. However, they are the largest type of granulocyte. They are responsible for inflammator ...
when cross-linked by antigen.
Type II hypersensitivity occurs when antibodies bind to antigens on the individual's own cells, marking them for destruction. This is also called antibody-dependent (or cytotoxic) hypersensitivity, and is mediated by
IgG
Immunoglobulin G (Ig G) is a type of antibody. Representing approximately 75% of serum antibodies in humans, IgG is the most common type of antibody found in blood circulation. IgG molecules are created and released by plasma B cells. Each IgG ...
and
IgM
Immunoglobulin M (IgM) is one of several isotypes of antibody (also known as immunoglobulin) that are produced by vertebrates. IgM is the largest antibody, and it is the first antibody to appear in the response to initial exposure to an antig ...
antibodies.
Immune complex
An immune complex, sometimes called an antigen-antibody complex or antigen-bound antibody, is a molecule formed from the binding of multiple antigens to antibodies. The bound antigen and antibody act as a unitary object, effectively an antigen o ...
es (aggregations of antigens, complement proteins, and IgG and IgM antibodies) deposited in various tissues trigger Type III hypersensitivity reactions.
Type IV hypersensitivity (also known as cell-mediated or ''delayed type hypersensitivity'') usually takes between two and three days to develop. Type IV reactions are involved in many autoimmune and infectious diseases, but may also involve
contact dermatitis
Contact dermatitis is a type of acute or chronic inflammation of the skin caused by exposure to chemical or physical agents. Symptoms of contact dermatitis can include itchy or dry skin, a red rash, bumps, blisters, or swelling. These rashes are ...
. These reactions are mediated by
T cell
A T cell is a type of lymphocyte. T cells are one of the important white blood cells of the immune system and play a central role in the adaptive immune response. T cells can be distinguished from other lymphocytes by the presence of a T-cell r ...
s,
monocyte
Monocytes are a type of leukocyte or white blood cell. They are the largest type of leukocyte in blood and can differentiate into macrophages and conventional dendritic cells. As a part of the vertebrate innate immune system monocytes also ...
s, and
macrophages.
Idiopathic inflammation
Inflammation is one of the first responses of the immune system to infection,
but it can appear without known cause.
Inflammation is produced by
eicosanoid
Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, around 20 carbon units in length. Eicosanoids are a s ...
s and
cytokine
Cytokines are a broad and loose category of small proteins (~5–25 kDa) important in cell signaling. Cytokines are peptides and cannot cross the lipid bilayer of cells to enter the cytoplasm. Cytokines have been shown to be involved in autocrin ...
s, which are released by injured or infected cells. Eicosanoids include
prostaglandins that produce fever and the
dilation of blood vessels
Vasodilation is the widening of blood vessels. It results from relaxation of smooth muscle cells within the vessel walls, in particular in the large veins, large arteries, and smaller arterioles. The process is the opposite of vasoconstriction ...
associated with inflammation, and
leukotriene
Leukotrienes are a family of eicosanoid inflammatory mediators produced in leukocytes by the oxidation of arachidonic acid (AA) and the essential fatty acid eicosapentaenoic acid (EPA) by the enzyme arachidonate 5-lipoxygenase.
Leukotrienes ...
s that attract certain white blood cells (leukocytes).
Common cytokines include
interleukin
Interleukins (ILs) are a group of cytokines (secreted proteins and signal molecules) that are expressed and secreted by white blood cells (leukocytes) as well as some other body cells. The human genome encodes more than 50 interleukins and related ...
s that are responsible for communication between white blood cells;
chemokine
Chemokines (), or chemotactic cytokines, are a family of small cytokines or signaling proteins secreted by cells that induce directional movement of leukocytes, as well as other cell types, including endothelial and epithelial cells. In additio ...
s that promote
chemotaxis; and
interferons that have anti-viral effects, such as shutting down
protein synthesis in the host cell.
Growth factor
A growth factor is a naturally occurring substance capable of stimulating cell proliferation, wound healing, and occasionally cellular differentiation. Usually it is a secreted protein or a steroid hormone. Growth factors are important for regul ...
s and cytotoxic factors may also be released. These cytokines and other chemicals recruit immune cells to the site of infection and promote healing of any damaged tissue following the removal of pathogens.
Manipulation in medicine

The immune response can be manipulated to suppress unwanted responses resulting from autoimmunity, allergy, and
transplant rejection
Transplant rejection occurs when transplanted tissue is rejected by the recipient's immune system, which destroys the transplanted tissue. Transplant rejection can be lessened by determining the molecular similitude between donor and recipient ...
, and to stimulate protective responses against pathogens that largely elude the immune system (see
immunization
Immunization, or immunisation, is the process by which an individual's immune system becomes fortified against an infectious agent (known as the immunogen).
When this system is exposed to molecules that are foreign to the body, called ''non-se ...
) or cancer.
Immunosuppression
Immunosuppressive drugs are used to control autoimmune disorders or
inflammation
Inflammation (from la, inflammatio) is part of the complex biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or irritants, and is a protective response involving immune cells, blood vessels, and molec ...
when excessive tissue damage occurs, and to prevent rejection after an
organ transplant
Organ transplantation is a medical procedure in which an organ (anatomy), organ is removed from one body and placed in the body of a recipient, to replace a damaged or missing organ. The donor and recipient may be at the same location, or organ ...
.
Anti-inflammatory
Anti-inflammatory is the property of a substance or treatment that reduces inflammation or swelling. Anti-inflammatory drugs, also called anti-inflammatories, make up about half of analgesics. These drugs remedy pain by reducing inflammation as o ...
drugs are often used to control the effects of inflammation.
Glucocorticoid
Glucocorticoids (or, less commonly, glucocorticosteroids) are a class of corticosteroids, which are a class of steroid hormones. Glucocorticoids are corticosteroids that bind to the glucocorticoid receptor that is present in almost every verteb ...
s are the most powerful of these drugs and can have many undesirable
side effects
In medicine, a side effect is an effect, whether therapeutic or adverse, that is secondary to the one intended; although the term is predominantly employed to describe adverse effects, it can also apply to beneficial, but unintended, consequence ...
, such as
central obesity
Abdominal obesity, also known as central obesity and truncal obesity, is a condition when excessive visceral fat around the stomach and abdomen has built up to the extent that it is likely to have a negative impact on health. Abdominal obesity has ...
,
hyperglycemia, and
osteoporosis. Their use is tightly controlled. Lower doses of anti-inflammatory drugs are often used in conjunction with cytotoxic or immunosuppressive drugs such as
methotrexate or
azathioprine
Azathioprine (AZA), sold under the brand name Imuran, among others, is an immunosuppressive medication. It is used in rheumatoid arthritis, granulomatosis with polyangiitis, Crohn's disease, ulcerative colitis, and systemic lupus erythematosus, ...
.
Cytotoxic drugs
Chemotherapy (often abbreviated to chemo and sometimes CTX or CTx) is a type of cancer treatment that uses one or more anti-cancer drugs (chemotherapeutic agents or alkylating agents) as part of a standardized chemotherapy regimen. Chemotherap ...
inhibit the immune response by killing dividing cells such as activated T cells. This killing is indiscriminate and other
constantly dividing cells and their organs are affected, which causes toxic side effects.
Immunosuppressive drugs such as
cyclosporin
Ciclosporin, also spelled cyclosporine and cyclosporin, is a calcineurin inhibitor, used as an immunosuppressant medication. It is a natural product. It is taken orally or intravenously for rheumatoid arthritis, psoriasis, Crohn's disea ...
prevent T cells from responding to signals correctly by inhibiting
signal transduction pathways.
Immunostimulation
Claims made by marketers of various products and
alternative health providers, such as
chiropractors
Chiropractic is a form of alternative medicine concerned with the diagnosis, treatment and prevention of mechanical disorders of the musculoskeletal system, especially of the spine. It has esoteric origins and is based on several pseudosc ...
,
homeopaths
Homeopathy or homoeopathy is a pseudoscientific system of alternative medicine. It was conceived in 1796 by the German physician Samuel Hahnemann. Its practitioners, called homeopaths, believe that a substance that causes symptoms of a di ...
, and
acupuncturists
Acupuncture is a form of alternative medicine and a component of traditional Chinese medicine (TCM) in which thin needles are inserted into the body. Acupuncture is a pseudoscience; the theories and practices of TCM are not based on scientif ...
to be able to stimulate or "boost" the immune system generally lack meaningful explanation and evidence of effectiveness.
Vaccination

Long-term ''active'' memory is acquired following infection by activation of B and T cells. Active immunity can also be generated artificially, through
vaccination
Vaccination is the administration of a vaccine to help the immune system develop immunity from a disease. Vaccines contain a microorganism or virus in a weakened, live or killed state, or proteins or toxins from the organism. In stimulating ...
. The principle behind vaccination (also called
immunization
Immunization, or immunisation, is the process by which an individual's immune system becomes fortified against an infectious agent (known as the immunogen).
When this system is exposed to molecules that are foreign to the body, called ''non-se ...
) is to introduce an antigen from a pathogen to stimulate the immune system and develop specific immunity against that particular pathogen without causing disease associated with that organism. This deliberate induction of an immune response is successful because it exploits the natural specificity of the immune system, as well as its inducibility. With infectious disease remaining one of the leading causes of death in the human population, vaccination represents the most effective manipulation of the immune system mankind has developed.
Many vaccines are based on non-cellular life, acellular components of micro-organisms, including harmless toxin components. Since many antigens derived from acellular vaccines do not strongly induce the adaptive response, most bacterial vaccines are provided with additional Immunologic adjuvant, adjuvants that activate the
antigen-presenting cells of the innate immune system and maximize immunogenicity.
Tumor immunology
Another important role of the immune system is to identify and eliminate tumors. This is called immune surveillance. The ''transformed cells'' of tumors express antigen#Tumor antigens, antigens that are not found on normal cells. To the immune system, these antigens appear foreign, and their presence causes immune cells to attack the transformed tumor cells. The antigens expressed by tumors have several sources;
some are derived from oncogenic viruses like human papillomavirus, which causes cancer of the cervical cancer, cervix, vulva cancer, vulva, vaginal cancer, vagina, penis cancer, penis, anal cancer, anus, oropharynx, mouth, and throat,
while others are the organism's own proteins that occur at low levels in normal cells but reach high levels in tumor cells. One example is an enzyme called tyrosinase that, when expressed at high levels, transforms certain skin cells (for example, melanocytes) into tumors called melanomas.
A third possible source of tumor antigens are proteins normally important for regulating cell growth and survival, that commonly mutate into cancer inducing molecules called oncogenes.

The main response of the immune system to tumors is to destroy the abnormal cells using killer T cells, sometimes with the assistance of helper T cells.
Tumor antigens are presented on MHC class I molecules in a similar way to viral antigens. This allows killer T cells to recognize the tumor cell as abnormal.
NK cells also kill tumorous cells in a similar way, especially if the tumor cells have fewer MHC class I molecules on their surface than normal; this is a common phenomenon with tumors. Sometimes antibodies are generated against tumor cells allowing for their destruction by the
complement system.
Some tumors evade the immune system and go on to become cancers.
Tumor cells often have a reduced number of MHC class I molecules on their surface, thus avoiding detection by killer T cells.
Some tumor cells also release products that inhibit the immune response; for example by secreting the cytokine TGF beta, TGF-β, which suppresses the activity of
macrophages and
lymphocyte
A lymphocyte is a type of white blood cell (leukocyte) in the immune system of most vertebrates. Lymphocytes include natural killer cells (which function in cell-mediated, cytotoxic innate immunity), T cells (for cell-mediated, cytotoxic ad ...
s.
In addition, immune tolerance, immunological tolerance may develop against tumor antigens, so the immune system no longer attacks the tumor cells.
Paradoxically, macrophages can promote tumor growth when tumor cells send out cytokines that attract macrophages, which then generate cytokines and growth factors such as Tumor necrosis factor alpha, tumor-necrosis factor alpha that nurture tumor development or promote stem-cell-like plasticity.
In addition, a combination of hypoxia in the tumor and a cytokine produced by macrophages induces tumor cells to decrease production of a protein that blocks metastasis and thereby assists spread of cancer cells.
Anti-tumor M1 macrophages are recruited in early phases to tumor development but are progressively differentiated to M2 with pro-tumor effect, an immunosuppressor switch. The hypoxia reduces the cytokine production for the anti-tumor response and progressively macrophages acquire pro-tumor M2 functions driven by the tumor microenvironment, including IL-4 and IL-10. Cancer immunotherapy covers the medical ways to stimulate the immune system to attack cancer tumors.
Predicting immunogenicity
Some drugs can cause a neutralizing immune response, meaning that the immune system produces neutralizing antibodies that counteract the action of the drugs, particularly if the drugs are administered repeatedly, or in larger doses. This limits the effectiveness of drugs based on larger peptides and proteins (which are typically larger than 6000 Dalton (unit), Da).
In some cases, the drug itself is not immunogenic, but may be co-administered with an immunogenic compound, as is sometimes the case for paclitaxel, Taxol. Computational methods have been developed to predict the immunogenicity of peptides and proteins, which are particularly useful in designing therapeutic antibodies, assessing likely virulence of mutations in viral coat particles, and validation of proposed peptide-based drug treatments. Early techniques relied mainly on the observation that hydrophile, hydrophilic amino acids are overrepresented in epitope regions than hydrophobe, hydrophobic amino acids;
however, more recent developments rely on machine learning techniques using databases of existing known epitopes, usually on well-studied virus proteins, as a training set.
A publicly accessible database has been established for the cataloguing of epitopes from pathogens known to be recognizable by B cells.
The emerging field of bioinformatics-based studies of immunogenicity is referred to as ''immunoinformatics''.
Immunoproteomics is the study of large sets of proteins (proteomics) involved in the immune response.
Evolution and other mechanisms
Evolution of the immune system
It is likely that a multicomponent, adaptive immune system arose with the first vertebrates, as
invertebrate
Invertebrates are a paraphyletic group of animals that neither possess nor develop a vertebral column (commonly known as a ''backbone'' or ''spine''), derived from the notochord. This is a grouping including all animals apart from the chordate ...
s do not generate lymphocytes or an antibody-based humoral response.
Many species, however, use mechanisms that appear to be precursors of these aspects of vertebrate immunity. Immune systems appear even in the structurally simplest forms of life, with bacteria using a unique defense mechanism, called the restriction modification system to protect themselves from viral pathogens, called bacteriophages. Prokaryotes (
bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of prokaryotic microorganisms. Typically a few micrometr ...
and archea) also possess acquired immunity, through a system that uses CRISPR sequences to retain fragments of the genomes of phage that they have come into contact with in the past, which allows them to block virus replication through a form of RNA interference. Prokaryotes also possess other defense mechanisms. Offensive elements of the immune systems are also present in protist, unicellular eukaryotes, but studies of their roles in defense are few.
Pattern recognition receptors are proteins used by nearly all organisms to identify molecules associated with pathogens. Antimicrobial peptides called
defensins are an evolutionarily conserved component of the innate immune response found in all animals and plants, and represent the main form of invertebrate systemic immunity.
The
complement system and phagocytic cells are also used by most forms of invertebrate life. Ribonucleases and the RNA interference pathway are conserved across all eukaryotes, and are thought to play a role in the immune response to viruses.
Unlike animals, plants lack phagocytic cells, but many plant immune responses involve systemic chemical signals that are sent through a plant.
Individual plant cells respond to molecules associated with pathogens known as
pathogen-associated molecular patterns
Pathogen-associated molecular patterns (PAMPs) are small molecular motifs conserved within a class of microbes. They are recognized by toll-like receptors (TLRs) and other pattern recognition receptors (PRRs) in both plants and animals. A vast arra ...
or PAMPs. When a part of a plant becomes infected, the plant produces a localized hypersensitive response, whereby cells at the site of infection undergo rapid
apoptosis to prevent the spread of the disease to other parts of the plant. Systemic acquired resistance is a type of defensive response used by plants that renders the entire plant Disease resistance in fruit and vegetables, resistant to a particular infectious agent.
RNA interference, RNA silencing mechanisms are particularly important in this systemic response as they can block virus replication.
Alternative adaptive immune system
adaptive immune system#Evolution, Evolution of the adaptive immune system occurred in an ancestor of the jawed vertebrates. Many of the classical molecules of the adaptive immune system (for example, immunoglobulins and
T-cell receptor
The T-cell receptor (TCR) is a protein complex found on the surface of T cells, or T lymphocytes, that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. The binding b ...
s) exist only in jawed vertebrates. A distinct
lymphocyte
A lymphocyte is a type of white blood cell (leukocyte) in the immune system of most vertebrates. Lymphocytes include natural killer cells (which function in cell-mediated, cytotoxic innate immunity), T cells (for cell-mediated, cytotoxic ad ...
-derived molecule has been discovered in primitive agnatha, jawless vertebrates, such as the lamprey and hagfish. These animals possess a large array of molecules called Variable lymphocyte receptors (VLRs) that, like the antigen receptors of jawed vertebrates, are produced from only a small number (one or two) of genes. These molecules are believed to bind pathogenic antigens in a similar way to
antibodies, and with the same degree of specificity.
Manipulation by pathogens
The success of any pathogen depends on its ability to elude host immune responses. Therefore, pathogens evolved several methods that allow them to successfully infect a host, while evading detection or destruction by the immune system.
Bacteria often overcome physical barriers by secreting enzymes that digest the barrier, for example, by using a type II secretion system. Alternatively, using a type III secretion system, they may insert a hollow tube into the host cell, providing a direct route for proteins to move from the pathogen to the host. These proteins are often used to shut down host defenses.
An evasion strategy used by several pathogens to avoid the innate immune system is to hide within the cells of their host (also called intracellular pathogenesis). Here, a pathogen spends most of its Biological life cycle, life-cycle inside host cells, where it is shielded from direct contact with immune cells, antibodies and complement. Some examples of intracellular pathogens include viruses, the foodborne illness, food poisoning bacterium ''Salmonella'' and the eukaryote, eukaryotic parasites that cause malaria (''Plasmodium spp.'') and leishmaniasis (''Leishmania spp.''). Other bacteria, such as ''Mycobacterium tuberculosis'', live inside a protective capsule that prevents lysis by complement. Many pathogens secrete compounds that diminish or misdirect the host's immune response.
Some bacteria form biofilms to protect themselves from the cells and proteins of the immune system. Such biofilms are present in many successful infections, such as the chronic ''Pseudomonas aeruginosa'' and ''Burkholderia cenocepacia'' infections characteristic of cystic fibrosis. Other bacteria generate surface proteins that bind to antibodies, rendering them ineffective; examples include ''Streptococcus'' (protein G), ''Staphylococcus aureus'' (protein A), and ''Peptostreptococcus magnus'' (protein L).
The mechanisms used to evade the adaptive immune system are more complicated. The simplest approach is to rapidly change non-essential epitopes (amino acids and/or sugars) on the surface of the pathogen, while keeping essential epitopes concealed. This is called antigenic variation. An example is HIV, which mutates rapidly, so the proteins on its viral envelope that are essential for entry into its host target cell are constantly changing. These frequent changes in antigens may explain the failures of vaccines directed at this virus. The parasite ''Trypanosoma brucei'' uses a similar strategy, constantly switching one type of surface protein for another, allowing it to stay one step ahead of the antibody response. Masking antigens with host molecules is another common strategy for avoiding detection by the immune system. In HIV, the envelope that covers the virus, virion is formed from the outermost membrane of the host cell; such "self-cloaked" viruses make it difficult for the immune system to identify them as "non-self" structures.
History of immunology

Immunology
Immunology is a branch of medicineImmunology for Medical Students, Roderick Nairn, Matthew Helbert, Mosby, 2007 and biology that covers the medical study of immune systems in humans, animals, plants and sapient species. In such we can see the ...
is a science that examines the structure and function of the immune system. It originates from medicine and early studies on the causes of immunity to disease. The earliest known reference to immunity was during the plague of Athens in 430 BC. Thucydides noted that people who had recovered from a previous bout of the disease could nurse the sick without contracting the illness a second time. In the 18th century, Pierre Louis Maupertuis, Pierre-Louis Moreau de Maupertuis experimented with scorpion venom and observed that certain dogs and mice were immune to this venom. In the 10th century, Persian physician Muhammad ibn Zakariya al-Razi, al-Razi (also known as Rhazes) wrote the first recorded theory of acquired immunity,
noting that a smallpox bout protected its survivors from future infections. Although he explained the immunity in terms of "excess moisture" being expelled from the blood—therefore preventing a second occurrence of the disease—this theory explained many observations about smallpox known during this time.
These and other observations of acquired immunity were later exploited by Louis Pasteur in his development of vaccination and his proposed germ theory of disease. Pasteur's theory was in direct opposition to contemporary theories of disease, such as the miasma theory of disease, miasma theory. It was not until Robert Koch's 1891 Koch's postulates, proofs, for which he was awarded a Nobel Prize in Physiology or Medicine, Nobel Prize in 1905, that microorganisms were confirmed as the cause of infectious disease. Viruses were confirmed as human pathogens in 1901, with the discovery of the yellow fever virus by Walter Reed.
Immunology made a great advance towards the end of the 19th century, through rapid developments in the study of humoral immunity and Cell-mediated immunity, cellular immunity.
Particularly important was the work of Paul Ehrlich, who proposed the side-chain theory to explain the specificity of the antigen-antibody reaction; his contributions to the understanding of humoral immunity were recognized by the award of a joint Nobel Prize in 1908, along with the founder of cellular immunology, Elie Metchnikoff.
In 1974, Niels Kaj Jerne developed the immune network theory; he shared a Nobel Prize in 1984 with Georges J. F. Köhler and César Milstein for theories related to the immune system.
See also
* Fc receptor
* Immune system receptors
* Immunostimulator
* Neuroimmune system
* Original antigenic sin – when the immune system uses immunological memory upon encountering a slightly different pathogen
* Plant disease resistance
* Polyclonal response
* Antigen#Tumor antigens, Tumor antigens
References
Citations
General bibliography
*
*
*
*
*
*
*
*
*
*
*
*
*
*
Further reading
* (The book'
sourcesare only online.) A popular science explanation of the immune system.
External links
– from the University of South Carolina School of Medicine (undergraduate level)
{{Authority control
Immune system,
 The immune system is a network of biological processes that protects an
The immune system is a network of biological processes that protects an  Some
Some  The adaptive immune system evolved in early vertebrates and allows for a stronger immune response as well as
The adaptive immune system evolved in early vertebrates and allows for a stronger immune response as well as  A B cell identifies pathogens when antibodies on its surface bind to a specific foreign antigen. This antigen/antibody complex is taken up by the B cell and processed by proteolysis into
A B cell identifies pathogens when antibodies on its surface bind to a specific foreign antigen. This antigen/antibody complex is taken up by the B cell and processed by proteolysis into  During exercise there is an increase in circulating
During exercise there is an increase in circulating  Long-term ''active'' memory is acquired following infection by activation of B and T cells. Active immunity can also be generated artificially, through
Long-term ''active'' memory is acquired following infection by activation of B and T cells. Active immunity can also be generated artificially, through  The main response of the immune system to tumors is to destroy the abnormal cells using killer T cells, sometimes with the assistance of helper T cells. Tumor antigens are presented on MHC class I molecules in a similar way to viral antigens. This allows killer T cells to recognize the tumor cell as abnormal. NK cells also kill tumorous cells in a similar way, especially if the tumor cells have fewer MHC class I molecules on their surface than normal; this is a common phenomenon with tumors. Sometimes antibodies are generated against tumor cells allowing for their destruction by the complement system.
Some tumors evade the immune system and go on to become cancers. Tumor cells often have a reduced number of MHC class I molecules on their surface, thus avoiding detection by killer T cells. Some tumor cells also release products that inhibit the immune response; for example by secreting the cytokine TGF beta, TGF-β, which suppresses the activity of macrophages and
The main response of the immune system to tumors is to destroy the abnormal cells using killer T cells, sometimes with the assistance of helper T cells. Tumor antigens are presented on MHC class I molecules in a similar way to viral antigens. This allows killer T cells to recognize the tumor cell as abnormal. NK cells also kill tumorous cells in a similar way, especially if the tumor cells have fewer MHC class I molecules on their surface than normal; this is a common phenomenon with tumors. Sometimes antibodies are generated against tumor cells allowing for their destruction by the complement system.
Some tumors evade the immune system and go on to become cancers. Tumor cells often have a reduced number of MHC class I molecules on their surface, thus avoiding detection by killer T cells. Some tumor cells also release products that inhibit the immune response; for example by secreting the cytokine TGF beta, TGF-β, which suppresses the activity of macrophages and 