IMRAD on:
[Wikipedia]
[Google]
[Amazon]
In scientific writing, IMRAD or IMRaD () (Introduction, Methods, Results, and Discussion) is a common organizational structure (a document format). IMRaD is the most prominent norm for the structure of a
 Original research articles are typically structured in this basic order
*Introduction – Why was the study undertaken? What was the
Original research articles are typically structured in this basic order
*Introduction – Why was the study undertaken? What was the
biosharing.org
Several important webpages on this topic are: * NLM's list a
* The EQUATOR Network's list a
Reporting guidelines and journals: fact & fiction
* TRANSPOS
(Transparency in Scholarly Publishing for Open Scholarship Evolution)
“a grassroots initiative to build a crowdsourced database of journal policies,” allowing faster and easier lookup and comparison, and potentially spurring harmonization Relatedly, SHERPA provides compliance-checking tools, and
scientific journal
In academic publishing, a scientific journal is a periodical publication intended to further the progress of science, usually by reporting new research.
Content
Articles in scientific journals are mostly written by active scientists such ...
article of the original research
Research is "creative and systematic work undertaken to increase the stock of knowledge". It involves the collection, organization and analysis of evidence to increase understanding of a topic, characterized by a particular attentiveness t ...
type.
Overview
 Original research articles are typically structured in this basic order
*Introduction – Why was the study undertaken? What was the
Original research articles are typically structured in this basic order
*Introduction – Why was the study undertaken? What was the research question
A research question is "a question that a research project sets out to answer". Choosing a research question is an essential element of both quantitative and qualitative research. Investigation will require data collection and analysis, and the me ...
, the tested hypothesis
A hypothesis (plural hypotheses) is a proposed explanation for a phenomenon. For a hypothesis to be a scientific hypothesis, the scientific method requires that one can test it. Scientists generally base scientific hypotheses on previous obse ...
or the purpose of the research?
*Methods – When, where, and how was the study done? What materials were used or who was included in the study groups (patients, etc.)?
*Results – What answer was found to the research question; what did the study find? Was the tested hypothesis true?
*Discussion – What might the answer imply and why does it matter? How does it fit in with what other researchers have found? What are the perspectives for future research?
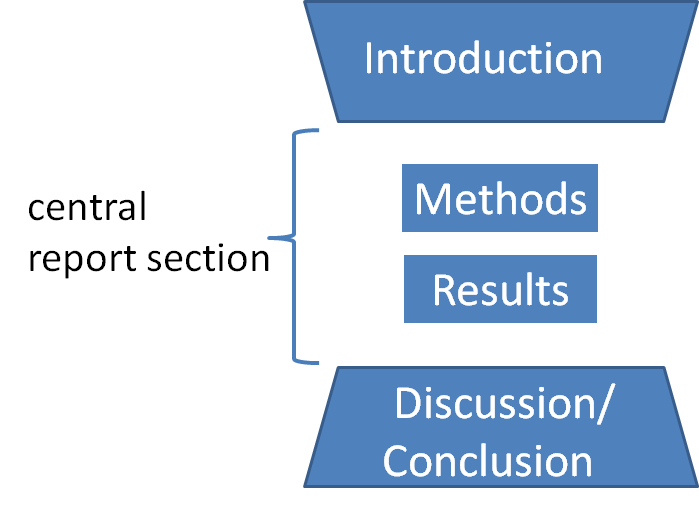
The plot and the flow of the story of the IMRaD style of writing are explained by a 'wine glass model' or hourglass model.
Writing, compliant with IMRaD format (IMRaD writing) typically first presents "(a) the subject that positions the study from the wide perspective", "(b) outline of the study", develops through "(c) study method", and "(d) the results", and concludes with "(e) outline and conclusion of the fruit of each topics", and "(f) the meaning of the study from the wide and general point of view". Here, (a) and (b) are mentioned in the section of the "Introduction", (c) and (d) are mentioned in the section of the "Method" and "Result" respectively, and (e) and (f) are mentioned in the section of the "Discussion" or "Conclusion".
In this sense, to explain how to line up the information in IMRaD writing, the 'wine glass model' (see the pattern diagram shown in Fig.1) will be helpful (see pp 2–3 of the Hilary Glasman-deal ). As mentioned in abovementioned textbook, the scheme of 'wine glass model' has two characteristics. The first one is "top-bottom symmetric shape", and the second one is "changing width" i.e. "the top is wide and it narrows towards the middle, and then widens again as it goes down toward the bottom".
The First one, "top-bottom symmetric shape", represents the symmetry of the story development. Note the shape of the top trapezoid (representing the structure of Introduction) and the shape of the trapezoid at the bottom are reversed. This is expressing that the same subject introduced in Introduction will be taken up again in suitable formation for the section of Discussion/Conclusion in these section in the reversed order. (See the relationship between abovementioned (a), (b) and (e), (f).)
The Second one, "the change of the width" of the schema shown in Fig.1, represents the change of generality of the view point. As along the flow of the story development, when the viewpoints are more general, the width of the diagram is expressed wider, and when they are more specialized and focused, the width is expressed narrower.
As the standard format of academic journals
The IMRAD format has been adopted by a steadily increasing number ofacademic journal
An academic journal or scholarly journal is a periodical publication in which scholarship relating to a particular academic discipline is published. Academic journals serve as permanent and transparent forums for the presentation, scrutiny, and ...
s since the first half of the 20th century. The IMRAD structure has come to dominate academic writing in the sciences, most notably in empirical
Empirical evidence for a proposition is evidence, i.e. what supports or counters this proposition, that is constituted by or accessible to sense experience or experimental procedure. Empirical evidence is of central importance to the sciences and ...
biomedicine. The structure of most public health journal articles reflects this trend. Although the IMRAD structure originates in the empirical sciences, it now also regularly appears in academic journals across a wide range of disciplines. Many scientific journals now not only prefer this structure but also use the IMRAD acronym as an instructional device in the instructions to their authors, recommending the use of the four terms as main headings. For example, it is explicitly recommended in the " Uniform Requirements for Manuscripts Submitted to Biomedical Journals" issued by the International Committee of Medical Journal Editors
The ICMJE recommendations (full title, "Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals") are a set of guidelines produced by the International Committee of Medical Journal Editors for stan ...
(previously called the '' Vancouver guidelines''): The text of observational and experimental articles is usually (but not necessarily) divided into the following sections: Introduction, Methods, Results, and Discussion. This so-called "IMRAD" structure is not an arbitrary publication format but rather a direct reflection of the process of scientific discovery. Long articles may need subheadings within some sections (especially Results and Discussion) to clarify their content. Other types of articles, such as case reports, reviews, and editorials, probably need to be formatted differently.The IMRAD structure is also recommended for empirical studies in the 6th edition of the publication manual of the
American Psychological Association
The American Psychological Association (APA) is the largest scientific and professional organization of psychologists in the United States, with over 133,000 members, including scientists, educators, clinicians, consultants, and students. It ha ...
( APA style). The APA publication manual is widely used by journals in the social
Social organisms, including human(s), live collectively in interacting populations. This interaction is considered social whether they are aware of it or not, and whether the exchange is voluntary or not.
Etymology
The word "social" derives from ...
, educational and behavioral sciences.
Benefits
The IMRAD structure has proved successful because it facilitates literature review, allowing readers to navigate articles more quickly to locate material relevant to their purpose. But the neat order of IMRAD rarely corresponds to the actual sequence of events or ideas of the research presented; the IMRAD structure effectively supports a reordering that eliminates unnecessary detail, and allows the reader to assess a well-ordered and noise-free presentation of the relevant and significant information. It allows the most relevant information to be presented clearly and logically to the readership, by summarizing the research process in an ideal sequence and without unnecessary detail.Caveats
The idealised sequence of the IMRAD structure has on occasion been criticised for being too rigid and simplistic. In a radio talk in 1964 theNobel laureate
The Nobel Prizes ( sv, Nobelpriset, no, Nobelprisen) are awarded annually by the Royal Swedish Academy of Sciences, the Swedish Academy, the Karolinska Institutet, and the Norwegian Nobel Committee to individuals and organizations who make o ...
Peter Medawar
Sir Peter Brian Medawar (; 28 February 1915 – 2 October 1987) was a Brazilian-British biologist and writer, whose works on graft rejection and the discovery of acquired immune tolerance have been fundamental to the medical practice of tissu ...
criticised this text structure for not giving a realistic representation of the thought processes of the writing scientist: "… the scientific paper may be a fraud because it misrepresents the processes of thought that accompanied or gave rise to the work that is described in the paper". Medawar's criticism was discussed at the XIXth General Assembly of the World Medical Association
The World Medical Association (WMA) is an international and independent confederation of free professional medical associations representing physicians worldwide. WMA was formally established on September 18, 1947 and has grown to 115 national m ...
in 1965. While respondents may argue that it is too much to ask from such a simple instructional device to carry the burden of representing the entire process of scientific discovery, Medawar's caveat expressed his belief that many students and faculty throughout academia treat the structure as a simple panacea. Medawar and others have given testimony both to the importance and to the limitations of the device.
Abstract considerations
In addition to the scientific article itself a brief abstract is usually required for publication. The abstract should, however, be composed to function as an autonomous text, even if some authors and readers may think of it as an almost integral part of the article. The increasing importance of well-formed autonomous abstracts may well be a consequence of the increasing use of searchable digital abstract archives, where a well-formed abstract will dramatically increase the probability for an article to be found by its optimal readership. Consequently, there is a strong recent trend toward developing formal requirements for abstracts, most often structured on the IMRAD pattern, and often with strict additional specifications of topical content items that should be considered for inclusion in the abstract. Such abstracts are often referred to as "structured abstracts". The growing importance of abstracts in the era of computerized literature search andinformation overload
Information overload (also known as infobesity, infoxication, information anxiety, and information explosion) is the difficulty in understanding an issue and effectively making decisions when one has too much information (TMI) about that issue, ...
has led some users to modify the IMRAD acronym to AIMRAD, in order to give due emphasis to the abstract.
Heading style variations
Usually, the IMRAD article sections use the IMRAD words as headings. A few variations can occur, as follows: * Many journals have a convention of omitting the "Introduction" heading, based on the idea that the reader who begins reading an article does not need to be told that the beginning of the text is the introduction. This print-era proscription is fading since the advent of the Web era, when having an explicit "Introduction" heading helps with navigation via document maps and collapsible/expandable TOC trees. (The same considerations are true regarding the presence or proscription of an explicit "Abstract" heading.) * In some journals, the "Methods" heading may vary, being "Methods and materials", "Materials and methods", or similar phrases. Some journals mandate that exactly the same wording for this heading be used for all articles without exception; other journals reasonably accept whatever each submitted manuscript contains, as long as it is one of these sensible variants. * The "Discussion" section may subsume any "Summary", "Conclusion", or "Conclusions" section, in which case there may or may not be any explicit "Summary", "Conclusion", or "Conclusions" subheading; or the "Summary"/"Conclusion"/"Conclusions" section may be a separate section, using an explicit heading on the same heading hierarchy level as the "Discussion" heading. Which of these variants to use as the default is a matter of each journal's chosen style, as is the question of whether the default style must be forced onto every article or whether sensible inter-article flexibility will be allowed. The journals which use the "Conclusion" or "Conclusions" along with a statement about the "Aim" or "Objective" of the study in the "Introduction" is following the newly proposed acronym "IaMRDC" which stands for "Introduction with aim, Materials and Methods, Results, Discussion, and Conclusion."Other elements that are typical although not part of the acronym
* Disclosure statements (''see main article at conflicts of interest in academic publishing'') ** Reader's theme that is the point of this element's existence: "Why should I (the reader) trust or believe what you (the author) say? Are you just making money off of saying it?" ** Appear either in opening footnotes or a section of the article body ** Subtypes of disclosure: *** Disclosure of funding (grants to the project) *** Disclosure of conflict of interest (grants to individuals, jobs/salaries, stock or stock options) * Clinical relevance statement ** Reader's theme that is the point of this element's existence: "Why should I (the reader) spend my time reading what you say? How is it relevant to my clinical practice? Basic research is nice, other people's cases are nice, but my time is triaged, so make your case for 'why bother'" ** Appear either as a display element (sidebar) or a section of the article body ** Format: short, a few sentences or bullet points * Ethical compliance statement ** Reader's theme that is the point of this element's existence: "Why should I believe that your study methods were ethical?" ** "We complied with theDeclaration of Helsinki
The Declaration of Helsinki (DoH, fi, Helsingin julistus, sv, Helsingforsdeklarationen) is a set of ethical principles regarding human experimentation developed originally in 1964 for the medical community by the World Medical Association (WMA ...
."
** "We got our study design approved by our local institutional review board
An institutional review board (IRB), also known as an independent ethics committee (IEC), ethical review board (ERB), or research ethics board (REB), is a committee that applies research ethics by reviewing the methods proposed for research to ens ...
before proceeding."
** "We got our study design approved by our local ethics committee before proceeding."
** "We treated our animals in accordance with our local Institutional Animal Care and Use Committee."
* Diversity, equity, and inclusion statement
** Reader's theme that is the point of this element's existence: "Why should I believe that your study methods consciously included people?" (for example, avoided inadvertently underrepresenting some people—participants or researchers—by race, ethnicity, sex, gender, or other factors)
** "We worked to ensure that people of color and transgender people were not underrepresented among the study population."
** "One or more of the authors of this paper self-identifies as living with a disability."
** "One or more of the authors of this paper self-identifies as transgender."
Additional standardization (reporting guidelines)
In the late 20th century and early 21st, the scientific communities found that the communicative value of journal articles was still much less than it could be ifbest practice
A best practice is a method or technique that has been generally accepted as superior to other known alternatives because it often produces results that are superior to those achieved by other means or because it has become a standard way of doing ...
s were developed, promoted, and enforced. Thus reporting guidelines (guidelines for how best to report information) arose. The general theme has been to create templates and checklist
A checklist is a type of job aid used in repetitive tasks to reduce failure by compensating for potential limits of human memory and attention. It helps to ensure consistency and completeness in carrying out a task. A basic example is the " to d ...
s with the message to the user being, "your article is not complete until you have done all of these things." In the 1970s, the ICMJE (International Committee of Medical Journal Editors) released the Uniform Requirements for Manuscripts Submitted to Biomedical Journals (Uniform Requirements or URM). Other such standards Standard may refer to:
Symbols
* Colours, standards and guidons, kinds of military signs
* Standard (emblem), a type of a large symbol or emblem used for identification
Norms, conventions or requirements
* Standard (metrology), an object t ...
, mostly developed in the 1990s through 2010s, are listed below. The academic medicine community is working hard on trying to raise compliance with good reporting standards, but there is still much to be done; for example, a 2016 review of instructions for authors in 27 emergency medicine journals found insufficient mention of reporting standards, and a 2018 study found that even when journals' instructions for authors mention reporting standards, there is a difference between a mention or badge
A badge is a device or accessory, often containing the insignia of an organization, which is presented or displayed to indicate some feat of service, a special accomplishment, a symbol of authority granted by taking an oath (e.g., police and ...
and enforcing the requirements that the mention or badge represents.
The advent of a need for best practices in data sharing
Data sharing is the practice of making data used for scholarly research available to other investigators. Many funding agencies, institutions, and publication venues have policies regarding data sharing because transparency and openness are consid ...
has expanded the scope of these efforts beyond merely the pages of the journal article itself. In fact, from the most rigorous versions of the evidence-based perspective, the distance to go is still quite formidable. FORCE11
FORCE11 is an international coalition of researchers, librarians, publishers and research funders working to reform or enhance the research publishing and communication system. Initiated in 2011 as a community of interest on scholarly communicatio ...
is an international coalition that has been developing standards for how to share research data sets properly and most effectively.
Most researchers cannot be familiar with all of the many reporting standards that now exist, but it is enough to know which ones must be followed in one's own work, and to know where to look for details when needed. Several organizations provide help with this task of checking one's own compliance with the latest standards:
* The EQUATOR Network
* The BioSharing collaborationbiosharing.org
Several important webpages on this topic are: * NLM's list a
* The EQUATOR Network's list a
Reporting guidelines and journals: fact & fiction
* TRANSPOS
(Transparency in Scholarly Publishing for Open Scholarship Evolution)
“a grassroots initiative to build a crowdsourced database of journal policies,” allowing faster and easier lookup and comparison, and potentially spurring harmonization Relatedly, SHERPA provides compliance-checking tools, and
AllTrials
AllTrials (sometimes called All Trials or AllTrials.net) is a project advocating that clinical research adopt the principles of open research. The project summarizes itself as "All trials registered, all results reported": that is, all clinical tri ...
provides a rallying point, for efforts to enforce openness
Openness is an overarching concept or philosophy that is characterized by an emphasis on transparency and collaboration. That is, openness refers to "accessibility of knowledge, technology and other resources; the transparency of action; the per ...
and completeness of clinical trial
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, diet ...
reporting. These efforts stand against publication bias
In published academic research, publication bias occurs when the outcome of an experiment or research study biases the decision to publish or otherwise distribute it. Publishing only results that show a significant finding disturbs the balance o ...
and against excessive corporate influence on scientific integrity.
See also
*Case report In medicine, a case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports may contain a demographic profile of the patient, but usually describe an unusual or novel occur ...
* Case series
A case series (also known as a clinical series) is a type of medical research study that tracks subjects with a known exposure, such as patients who have received a similar treatment, or examines their medical records for exposure and outcome. Cas ...
* Eight-legged essay
* Five paragraph essay
The five-paragraph essay is a format of essay having five paragraphs: one introductory paragraph, three body paragraphs with support and development, and one concluding paragraph. Because of this structure, it is also known as a hamburger essay ...
* IRAC
* Journal Article Tag Suite (JATS)
* Literature review
A literature review is an overview of the previously published works on a topic. The term can refer to a full scholarly paper or a section of a scholarly work such as a book, or an article. Either way, a literature review is supposed to provid ...
* Meta-analyses
A meta-analysis is a statistical analysis that combines the results of multiple scientific studies. Meta-analyses can be performed when there are multiple scientific studies addressing the same question, with each individual study reporting m ...
* Schaffer paragraph
The Jane Schaffer method is a formula for essay writing that is taught in some U.S. middle schools and high schools. Developed by a San Diego teacher named Jane Schaffer, who started offering training and a 45-day curriculum in 1995, it is in ...
References
{{reflist Writing Academic publishing Scientific documents Technical communication Style guides for technical and scientific writing Academic terminology Medical publishing