Biosimilars Patent Cliff.png on:
[Wikipedia]
[Google]
[Amazon]
A biosimilar (also known as follow-on biologic or subsequent entry biologic) is a
Library of Congress website
and search H.R. 1548 in 111th Congress Session. Since 2004 the FDA has held a series of public meetings on biosimilars. The FDA gained the authority to approve biosimilars (including interchangeables that are substitutable with their reference product) as part of the
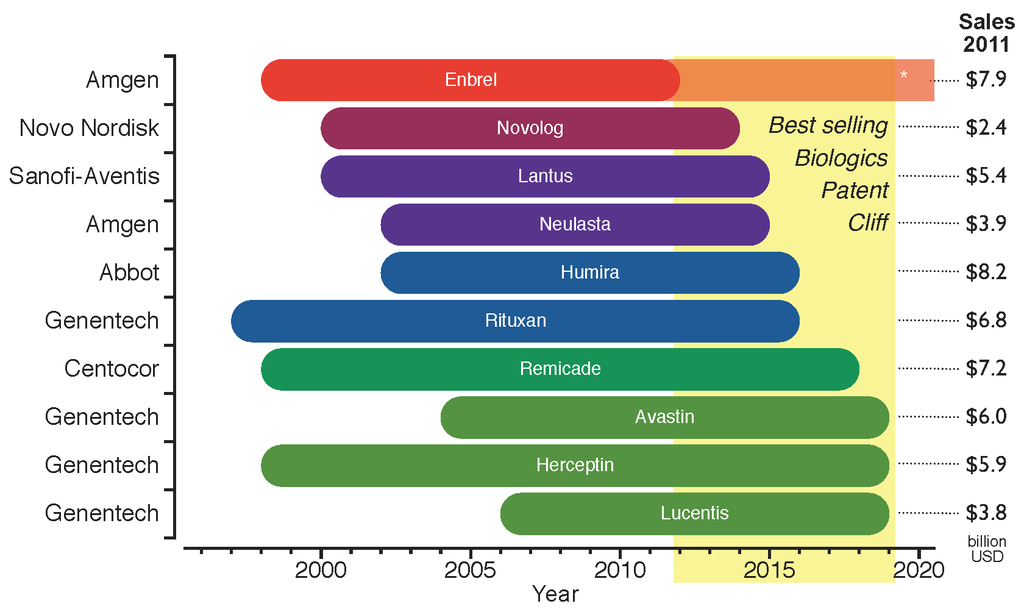 The legal requirements of approval pathways, together with the costly manufacturing processes, escalates the developing costs for biosimilars that could be between $75–$250 million per molecule. This market entry barrier affects not only the companies willing to produce them but could also delay availability of inexpensive alternatives for public healthcare institutions that subsidize treatment for their patients. Even though the biosimilars market is rising, the price drop for biological drugs at risk of patent expiration will not be as great as for other generic drugs; in fact it has been estimated that the price for biosimilar products will be 65%-85% of their originators. Biosimilars are drawing market's attention since there is an upcoming patent cliff, which will put nearly 36% of the $140 billion market for biologic drugs at risk (as of 2011), this considering only the top 10 selling products.
The global biosimilars market was in 2013, and is expected to reach by 2020, driven by the
The legal requirements of approval pathways, together with the costly manufacturing processes, escalates the developing costs for biosimilars that could be between $75–$250 million per molecule. This market entry barrier affects not only the companies willing to produce them but could also delay availability of inexpensive alternatives for public healthcare institutions that subsidize treatment for their patients. Even though the biosimilars market is rising, the price drop for biological drugs at risk of patent expiration will not be as great as for other generic drugs; in fact it has been estimated that the price for biosimilar products will be 65%-85% of their originators. Biosimilars are drawing market's attention since there is an upcoming patent cliff, which will put nearly 36% of the $140 billion market for biologic drugs at risk (as of 2011), this considering only the top 10 selling products.
The global biosimilars market was in 2013, and is expected to reach by 2020, driven by the
Lupin
and
Overview of Biosimilar Products
U.S. Food and Drug Administration
Biosimilar Regulatory Review and Approval
U.S. Food and Drug Administration
Interchangeable Biological Products
U.S. Food and Drug Administration {{Portal bar , Medicine Biotechnology Drugs Life sciences industry
biologic medical product
A biopharmaceutical, also known as a biological medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources. Different from totally synthesized pharmaceuticals, t ...
that is almost an identical copy of an original product that is manufactured by a different company. Biosimilars are officially approved versions of original "innovator" products and can be manufactured when the original product's patent expires. Reference to the innovator product is an integral component of the approval.
Unlike with generic drug
A generic drug is a pharmaceutical drug that contains the same chemical substance as a drug that was originally protected by chemical patents. Generic drugs are allowed for sale after the patents on the original drugs expire. Because the active ch ...
s of the more common small-molecule
Within the fields of molecular biology and pharmacology, a small molecule or micromolecule is a low molecular weight (≤ 1000 daltons) organic compound that may regulate a biological process, with a size on the order of 1 nm. Many drugs ar ...
type, biologics generally exhibit high molecular complexity and may be quite sensitive to changes in manufacturing processes. Despite that heterogeneity, all biopharmaceuticals, including biosimilars, must maintain consistent quality and clinical performance throughout their lifecycle.
Drug-related authorities such as the European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or Euro ...
(EMA) of the European Union, the United States Food and Drug Administration (FDA), and the Health Products and Food Branch The Health Products and Food Branch (HPFB) of Health Canada manages the health-related risks and benefits of health products and food by minimizing risk factors while maximizing the safety provided by the regulatory system and providing information ...
of Health Canada
Health Canada (HC; french: Santé Canada, SC)Health Canada is the applied title under the Federal Identity Program; the legal title is Department of Health (). is the Structure of the Canadian federal government#Departments, with subsidiary unit ...
hold their own guidance on requirements for demonstration of the similar nature of two biological products in terms of safety and efficacy. According to them, analytical studies demonstrate that the biological product is highly similar to the reference product, despite minor differences in clinically inactive components, animal studies (including the assessment of toxicity), and a clinical study or studies (including the assessment of immunogenicity and pharmacokinetics
Pharmacokinetics (from Ancient Greek ''pharmakon'' "drug" and ''kinetikos'' "moving, putting in motion"; see chemical kinetics), sometimes abbreviated as PK, is a branch of pharmacology dedicated to determining the fate of substances administered ...
or pharmacodynamics). They are sufficient to demonstrate safety, purity, and potency in one or more appropriate conditions of use for which the reference product is licensed and is intended to be used and for which licensure is sought for the biological product.
The World Health Organization (WHO) published its "Guidelines for the evaluation of similar biotherapeutic products (SBPs)" in 2009. The purpose of this guideline is to provide an international norm for evaluating biosimilars.
The EMA has granted marketing authorizations for more than 50 biosimilars since 2006, (first approved biosimilar Somatropin (Growth hormone)). The first biosimilar of a monoclonal antibody to be approved worldwide was a biosimilar of infliximab in the EU in 2013. On March 6, 2015, the FDA approved the United States' first biosimilar product, the biosimilar of filgrastim called filgrastim-sndz (trade name Zarxio) by Sandoz.
Approval processes
United States
In the United States, the Food and Drug Administration (FDA) held that new legislation was required to enable them to approve biosimilars to those biologics originally approved through the PHS Act pathway. Additional Congressional hearings have been held. On March 17, 2009, the Pathway for Biosimilars Act was introduced in the House. See thLibrary of Congress website
and search H.R. 1548 in 111th Congress Session. Since 2004 the FDA has held a series of public meetings on biosimilars. The FDA gained the authority to approve biosimilars (including interchangeables that are substitutable with their reference product) as part of the
Patient Protection and Affordable Care Act
The Affordable Care Act (ACA), formally known as the Patient Protection and Affordable Care Act and colloquially known as Obamacare, is a landmark U.S. federal statute enacted by the 111th United States Congress and signed into law by Presi ...
signed into law by President Obama on March 23, 2010.
The FDA has previously approved biologic products using comparability, for example, Omnitrope
Growth hormone therapy refers to the use of growth hormone (GH) as a prescription medication—it is one form of hormone therapy. Growth hormone is a peptide hormone secreted by the pituitary gland that stimulates growth and cell reproduction. ...
in May 2006, but this like Enoxaparin
Enoxaparin sodium, sold under the brand name Lovenox among others, is an anticoagulant medication (blood thinner). It is used to treat and prevent deep vein thrombosis (DVT) and pulmonary embolism (PE) including during pregnancy and following c ...
was also to a reference product, Genotropin
Growth hormone therapy refers to the use of growth hormone (GH) as a prescription medication—it is one form of hormone therapy. Growth hormone is a peptide hormone secreted by the pituitary gland that stimulates growth and cell reproduction. I ...
, originally approved as a biologic drug Biologic may refer to:
* biology
Biology is the scientific study of life. It is a natural science with a broad scope but has several unifying themes that tie it together as a single, coherent field. For instance, all organisms are made up ...
under the FD&C Act.
On March 6, 2015, Zarxio
Filgrastim, sold under the brand name Neupogen among others, is a medication used to treat low neutrophil count. Low neutrophil counts may occur with HIV/AIDS, following chemotherapy or radiation poisoning, or be of an unknown cause. It may ...
obtained the first approval of FDA. Sandoz's Zarxio is biosimilar to Amgen's Neupogen (filgrastim), which was originally licensed in 1991. This is the first product to be passed under the Biologics Price Competition and Innovation Act of 2009 (BPCI Act), which was passed as part of the Affordable Healthcare Act. But Zarxio was approved as a biosimilar, not as an interchangeable product, the FDA notes. And under the BPCI Act, only a biologic that has been approved as an "interchangeable" may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product. The FDA said its approval of Zarxio is based on review of evidence that included structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data and other clinical safety and effectiveness data that demonstrates Zarxio
Filgrastim, sold under the brand name Neupogen among others, is a medication used to treat low neutrophil count. Low neutrophil counts may occur with HIV/AIDS, following chemotherapy or radiation poisoning, or be of an unknown cause. It may ...
is biosimilar to Neupogen.
In March 2020, most protein products that were approved as drug products (including every insulin currently on the market ) are scheduled to open up to biosimilar and interchangeable competition in the United States. However, "chemically synthesized polypeptides" are excluded from this transition, which means that a product that falls within this category won't be able to come to market as a biosimilar or interchangeable product, but will have to come to the market under a different pathway.
Background
Cloning of human genetic material and development of in vitro biological production systems has allowed the production of virtually any recombinant DNA based biological substance for eventual development of a drug. Monoclonal antibody technology combined with recombinant DNA technology has paved the way for tailor-made and targeted medicines. Gene- and cell-based therapies are emerging as new approaches. Recombinant therapeuticproteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
are of a complex nature (composed of a long chain of amino acids, modified amino acids, derivatized by sugar moieties, folded by complex mechanisms). These proteins are made in living cells (bacteria, yeast, animal or human cell lines). The ultimate characteristics of a drug containing a recombinant therapeutic protein are to a large part determined by the process through which they are produced: choice of the cell type, development of the genetically modified cell for production, production process, purification process, formulation of the therapeutic protein into a drug.
After the expiry of the patent of approved recombinant drugs (e.g., insulin
Insulin (, from Latin ''insula'', 'island') is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the ''INS'' gene. It is considered to be the main anabolic hormone of the body. It regulates the metabolism o ...
, human growth hormone, interferons, erythropoietin
Erythropoietin (; EPO), also known as erythropoetin, haematopoietin, or haemopoietin, is a glycoprotein cytokine secreted mainly by the kidneys in response to cellular hypoxia; it stimulates red blood cell production (erythropoiesis) in the bo ...
, monoclonal antibodies and more) any other biotech company can develop and market these biologics
A biopharmaceutical, also known as a biological medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources. Different from totally synthesized pharmaceuticals, th ...
(thus called biosimilars).
The typical reference product has undergone numerous changes in its manufacturing processes, and such changes in the manufacturing process (ranging from a change in the supplier of cell culture media to new purification methods or new manufacturing sites) was substantiated with appropriate data and was approved by the EMA.
The current concept of development of biosimilar mAbs
A monoclonal antibody (mAb, more rarely called moAb) is an antibody produced from a cell Lineage (evolution), Lineage made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell.
Mon ...
follows the principle that an extensive state of the art physicochemical, analytical and functional comparison of the molecules is complemented by comparative non-clinical and clinical data that establish equivalent efficacy and safety in a clinical "model" indication that is most sensitive to detect any minor differences (if these exist) between the biosimilar and its reference mAb also at the clinical level.
The EMA has recognized this fact, which has resulted in the establishment of the term "biosimilar" in recognition that, whilst biosimilar products are similar to the original product, they are not exactly the same.
Originally the complexity of biological molecules led to requests for substantial efficacy and safety data for a biosimilar approval. This has been progressively replaced with a greater dependence on assays, from quality through to clinical, that show assay sensitivity sufficient to detect any significant difference in dose. However, the safe application of biologics depends on an informed and appropriate use by healthcare professionals and patients. Introduction of biosimilars also requires a specifically designed pharmacovigilance plan. It is difficult and costly to recreate biologics because the complex proteins are derived from living organisms that are genetically modified. In contrast, small molecule drugs made up of a chemically based compound can be easily replicated and are considerably less expensive to reproduce. In order to be released to the public, biosimilars must be shown to be as close to identical to the parent innovator biologic product based on data compiled through clinical, animal, analytical studies and conformational status.
Generally, once a drug is released in the market by the FDA, it has to be re-evaluated for its safety and efficacy once every six months for the first and second years. Afterward, re-evaluations are conducted yearly, and the result of the assessment should be reported to authorities such as FDA. Biosimilars are required to undergo pharmacovigilance (PVG) regulations as its reference product. Thus biosimilars approved by the EMA are required to submit a risk management plan (RMP) along with the marketing application and have to provide regular safety update reports after the product is in the market. The RMP includes the safety profile of the drug and proposes the prospective pharmacovigilance studies.
Several PK studies, such as studies conducted by Committee for Medicinal Products for Human Use (CHMP), have been conducted under various ranges of conditions; Antibodies from an originator's product versus antibodies from a biosimilar; combination therapy and monotherapy; various diseases, etc. on the purpose to verify comparability in pharmacokinetics of the biosimilar with the reference medicinal product in a sufficiently sensitive and homogeneous population.
Nomenclature
In the European Union, no unique identifier of a biosimilar medicine product is required, as the same rules are followed as for all biologics. The US decided on a different approach, requiring the assignment of a four-letter suffix to the nonproprietary name of the original product to distinguish between innovator drugs and their biosimilars. Japan has similar requirements. The suffix approach has been criticized on the grounds of compromising the INN system and delaying the marketing of biosimilars. Australia decided not to use a 4-letter suffix. A version of the four-letter suffix has been proposed to the WHO as the biological qualifier (BQ). It is not part of the international nonproprietary name (INN), but is proposed to be managed under the same registry. The report 1 of the May 2017 WHO Expert Consultation on Improving Access to and Use of Similar Biotherapeutic Products, published in October 2017, revealed on page 4, that following the outcome arising from the meeting: "No consensus was reached on whether WHO should continue with the BQ… WHO will not be proceeding with this at present."Australia
Biosimilars available in Australia include adalimumab, bevacizumab, enoxaparin, epoetin lambda, etanercept, filgrastim, follitropin alfa, infliximab, insulin aspart, insulin glargine, pegfilgrastim, rituximab, teriparatide, and trastuzumab.European Union
Biosimilar medicines approved in the European Union (EU) are interchangeable with their reference medicine or with an equivalent biosimilar.United States
BPCI Act
TheBiologics Price Competition and Innovation Act
A biopharmaceutical, also known as a biological medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources. Different from totally synthesized pharmaceuticals, t ...
of 2009 (BPCI Act) was originally sponsored and introduced on June 26, 2007, by Senator Edward Kennedy (D-MA). It was formally passed under the Patient Protection and Affordable Care Act
The Affordable Care Act (ACA), formally known as the Patient Protection and Affordable Care Act and colloquially known as Obamacare, is a landmark U.S. federal statute enacted by the 111th United States Congress and signed into law by Presi ...
(PPAC Act), signed into law by President Barack Obama on March 23, 2010. The BPCI Act was an amendment to the Public Health Service Act (PHS Act) to create an abbreviated approval pathway for biological products that are demonstrated to be highly similar (biosimilar) to a Food and Drug Administration (FDA) approved biological product. The BPCI Act is similar, conceptually, to the Drug Price Competition and Patent Term Restoration Act of 1984 (also referred to as the "Hatch-Waxman Act") which created biological drug approval through the Federal Food, Drug, and Cosmetic Act (FFD&C Act). The BPCI Act aligns with the FDA's longstanding policy of permitting appropriate reliance on what is already known about a drug, thereby saving time and resources and avoiding unnecessary duplication of human or animal testing. The FDA has released a total of four draft guidelines related to biosimilar or follow-on biologics development. Upon the release of the first three guidance documents the FDA held a public hearing on May 11, 2012.
In 2018, the FDA released a Biosimilars Action Plan to implement regulations from the BPCI, including limiting the abuse of the Risk Evaluation and Mitigation Strategy (REMS) system for evergreening
Evergreening is any of various legal, business, and technological strategies by which producers (often pharmaceutical companies) extend the lifetime of their patents that are about to expire in order to retain revenues from them. Often the practice ...
and transitioning insulin and human growth hormone to regulation as biologics rather than drugs.
US approved biosimilars
Proposed reforms
In the United States, biosimilars have not had the expected impact on prices, leading a 2019 proposal to price regulate instead after an exclusivity period. Another proposal requires originators to share the underlying cell lines. In 2019, the proposed Biologic Patent Transparency Act would help addressevergreening
Evergreening is any of various legal, business, and technological strategies by which producers (often pharmaceutical companies) extend the lifetime of their patents that are about to expire in order to retain revenues from them. Often the practice ...
" patent thickets" by requiring that all patents protecting a biosimilar be disclosed.
Biosimilars have found it difficult to get market share, which led biosimilar developer Pfizer to sue Johnson & Johnson
Johnson & Johnson (J&J) is an American multinational corporation founded in 1886 that develops medical devices, pharmaceuticals, and consumer packaged goods. Its common stock is a component of the Dow Jones Industrial Average and the company i ...
over anticompetitive contracts with pharmacy benefit managers which bundle
Bundle or Bundling may refer to:
* Bundling (packaging), the process of using straps to bundle up items
Biology
* Bundle of His, a collection of heart muscle cells specialized for electrical conduction
* Bundle of Kent, an extra conduction pat ...
discounts; these are sometimes called the "rebate wall", and the rebates are generally unavailable to customers.
Market implications
 The legal requirements of approval pathways, together with the costly manufacturing processes, escalates the developing costs for biosimilars that could be between $75–$250 million per molecule. This market entry barrier affects not only the companies willing to produce them but could also delay availability of inexpensive alternatives for public healthcare institutions that subsidize treatment for their patients. Even though the biosimilars market is rising, the price drop for biological drugs at risk of patent expiration will not be as great as for other generic drugs; in fact it has been estimated that the price for biosimilar products will be 65%-85% of their originators. Biosimilars are drawing market's attention since there is an upcoming patent cliff, which will put nearly 36% of the $140 billion market for biologic drugs at risk (as of 2011), this considering only the top 10 selling products.
The global biosimilars market was in 2013, and is expected to reach by 2020, driven by the
The legal requirements of approval pathways, together with the costly manufacturing processes, escalates the developing costs for biosimilars that could be between $75–$250 million per molecule. This market entry barrier affects not only the companies willing to produce them but could also delay availability of inexpensive alternatives for public healthcare institutions that subsidize treatment for their patients. Even though the biosimilars market is rising, the price drop for biological drugs at risk of patent expiration will not be as great as for other generic drugs; in fact it has been estimated that the price for biosimilar products will be 65%-85% of their originators. Biosimilars are drawing market's attention since there is an upcoming patent cliff, which will put nearly 36% of the $140 billion market for biologic drugs at risk (as of 2011), this considering only the top 10 selling products.
The global biosimilars market was in 2013, and is expected to reach by 2020, driven by the patent expiration The term of a patent is the maximum time during which it can be maintained in force. It is usually expressed in a number of years either starting from the filing date of the patent application or from the date of grant of the patent. In most patent ...
of additional ten blockbuster biologic drugs.
Companies
Certain companies (in some cases subsidiaries) tend to operate as generic drug manufacturers, with major ones includingTeva
Teva is the Hebrew word for nature ( he, טבע, "nature").
Teva may refer to:
Companies
* Teva Footwear, American footwear manufacturer
* Teva Naot, Israeli footwear manufacturer
* Teva Pharmaceuticals, Israeli multinational pharmaceutical com ...
, Mylan, and Sandoz and may also extend that focus to biosimilars. Sandoz, for example, introduced the first biosimilar in the United States, and plans to introduce another in 2020. Newer companies such as India-based Cadila Pharmaceuticals
Cadila Pharmaceuticals is an Indian multinational pharmaceutical company based in Ahmedabad, Gujarat
Gujarat (, ) is a state along the western coast of India. Its coastline of about is the longest in the country, most of which lies o ...
, Sun Pharma
Sun Pharmaceutical Industries Limited (d/b/a Sun Pharma) is an Indian multinational pharmaceutical company headquartered in Mumbai, that manufactures and sells pharmaceutical formulations and active pharmaceutical ingredients (APIs) in more th ...
, Aurobindo Pharma
Aurobindo Pharma Limited is an Indian multinational pharmaceutical manufacturing company headquartered in HITEC City, Hyderabad, India. The company manufactures generic pharmaceuticals and active pharmaceutical ingredients. The company’s a ...
Lupin
and
Dr. Reddy's Laboratories
Dr. Reddy's Laboratories is an Indian multinational pharmaceutical company based in Hyderabad. The company was founded by Kallam Anji Reddy, who previously worked in the mentor institute Indian Drugs and Pharmaceuticals Limited. Dr. Reddy's m ...
as well as Canada-based Apotex have taken share in traditional generics, which has led older companies to shift their focus to complex drugs such as biosimilars.
References
Further reading
* * * * *External links
Overview of Biosimilar Products
U.S. Food and Drug Administration
Biosimilar Regulatory Review and Approval
U.S. Food and Drug Administration
Interchangeable Biological Products
U.S. Food and Drug Administration {{Portal bar , Medicine Biotechnology Drugs Life sciences industry