|
Apotex
Apotex Inc. is a Canadian pharmaceutical corporation. Founded in 1974 by Barry Sherman, the company is the largest producer of generic drugs in Canada, with annual sales exceeding . By 2016, Apotex employed over 10,000 people as one of Canada's largest drug manufacturers, with over 300 products selling in over 115 countries. Revenues were about CA$1.19 billion annually. Apotex manufactures and distributes generic medications for a range of diseases and health conditions that include cancer, diabetes, high cholesterol, glaucoma, infections and blood pressure. Apotex is a member of the Canadian Generic Pharmaceutical Association (CGPA), the Generic Pharmaceutical Association (GPhA), an associate member of the Canadian Animal Health Institute (CAHI), the Canadian Association for Pharmacy Distribution Management (CAPDM), as well as the Greater Toronto Area's Partners in Project Green. History Apotex began with limited staff in a 5,000-square-foot warehouse. When Barry Sherman started ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barry Sherman
Bernard Charles "Barry" Sherman, (February 25, 1942 – December 13, 2017) was a Canadian businessman and philanthropist who was chairman and CEO of Apotex Inc. With an estimated net worth of US$3.2 billion at the time of his death, according to ''Forbes'', Sherman was the 12th-wealthiest man in Canada. Another publication, ''Canadian Business'', stated his fortune at CA$4.77 billion, ranking him the 15th richest man in Canada. Sherman, a University of Toronto graduate with a doctorate in engineering from the Massachusetts Institute of Technology, got his start in the pharmaceutical business in the 1960s, when the estate of his uncle Louis Lloyd Winter let him run Empire Laboratories, the late uncle's drug company. This eventually led Sherman to form Apotex, where he earned a reputation among both competitors and government regulators for extreme combativeness, often including litigation. Sherman's four cousins, who were supposed to have received five-percent stakes in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sanofi-Aventis
Sanofi S.A. is a French multinational pharmaceutical and healthcare company headquartered in Paris, France. Originally, the corporation was established in 1973 and merged with Synthélabo in 1999 to form Sanofi-Synthélabo. In 2004, Sanofi-Synthélabo merged with Aventis and renamed to Sanofi-Aventis, which were each the product of several previous mergers. It changed its name back to Sanofi in May 2011. The company is a component of the Euro Stoxx 50 stock market index. Sanofi engages in the research and development, manufacturing and marketing of pharmaceutical drugs principally in the prescription market, but the firm also develops over-the-counter medication. The corporation covers seven major therapeutic areas: cardiovascular, central nervous system, diabetes, internal medicine, oncology, thrombosis and vaccines (it is the world's largest producer of the latter through its subsidiary Sanofi Pasteur). History Sanofi-Synthélabo Sanofi was founded in 1973 as a subsidiar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Generic Drug
A generic drug is a pharmaceutical drug that contains the same chemical substance as a drug that was originally protected by chemical patents. Generic drugs are allowed for sale after the patents on the original drugs expire. Because the active chemical substance is the same, the medical profile of generics is equivalent in performance. A generic drug has the same active pharmaceutical ingredient (API) as the original, but it may differ in some characteristics such as the manufacturing process, formulation, excipients, color, taste, and packaging. Although they may not be associated with a particular company, generic drugs are usually subject to government regulations in the countries in which they are dispensed. They are labeled with the name of the manufacturer and a generic non-proprietary name such as the United States Adopted Name (USAN) or International Nonproprietary Name (INN) of the drug. A generic drug must contain the same active ingredients as the original brand-name ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Generic Drug
A generic drug is a pharmaceutical drug that contains the same chemical substance as a drug that was originally protected by chemical patents. Generic drugs are allowed for sale after the patents on the original drugs expire. Because the active chemical substance is the same, the medical profile of generics is equivalent in performance. A generic drug has the same active pharmaceutical ingredient (API) as the original, but it may differ in some characteristics such as the manufacturing process, formulation, excipients, color, taste, and packaging. Although they may not be associated with a particular company, generic drugs are usually subject to government regulations in the countries in which they are dispensed. They are labeled with the name of the manufacturer and a generic non-proprietary name such as the United States Adopted Name (USAN) or International Nonproprietary Name (INN) of the drug. A generic drug must contain the same active ingredients as the original brand-name ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plavix
Clopidogrel — sold under the brand name Plavix, among others — is an antiplatelet drug, antiplatelet medication used to reduce the risk of Cardiovascular disease, heart disease and stroke in those at high risk. It is also used together with aspirin in myocardial infarction, heart attacks and following the placement of a Coronary stent, coronary artery stent (management of acute coronary syndrome#Antiplatelet drugs, dual antiplatelet therapy). It is taken Oral administration, by mouth. Its effect starts about two hours after intake and lasts for five days. Common side effects include headache, nausea, easy bruising, itching, and heartburn. More severe side effects include bleeding and thrombotic thrombocytopenic purpura. While there is no evidence of harm from use during pregnancy, such use has not been well studied. Clopidogrel is in the thienopyridine-class of antiplatelets. It works by irreversibly inhibiting a receptor called P2Y12, P2Y12 on platelets. Clopidogrel was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
North York
North York is one of the six administrative districts of Toronto, Ontario, Canada. It is located directly north of York, Old Toronto and East York, between Etobicoke to the west and Scarborough to the east. As of the 2016 Census, it had a population of 869,401. North York was created as a township in 1922 out of the northern part of the former township of York, a municipality that was located along the western border of Old Toronto. Following its inclusion in Metropolitan Toronto in 1953, it was one of the fastest-growing parts of the region due to its proximity to Old Toronto. It was declared a borough in 1967, and later became a city in 1979, attracting high-density residences, rapid transit, and a number of corporate headquarters in North York City Centre, its central business district. In 1998, North York was amalgamated with the rest of Metropolitan Toronto to form the new city of Toronto and has since been a secondary economic hub of the city outside Downtown Toronto. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cangene Corp
Cangene Corporation was a biopharmaceutical company based in Winnipeg, Manitoba, Canada. It was founded in 1984 and specialized in hyperimmunes, contract manufacturing, biopharmaceuticals and biodefense. Cangene was 61% owned by Canadian pharmaceutical giant Apotex and was publicly listed on the TSX under the symbol CNJ. Business model Cangene's business model shifted several times during its existence. There is some consensus that the company came to rely on revenue from United States stockpiling and bioterrorism contracts and did not adequately prepare for the disappearance of this revenue. At one point, the company attempted a shift away from contract manufacturing to research & development, but abandoned this track after about two years and subsequently moved to marketing of ready-for-launch products developed by other companies. History Cangene was founded in 1984 and had an employee count of 650 in 2004. In 2009, the number of personnel in the Winnipeg facilities alone w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biosimilar
A biosimilar (also known as follow-on biologic or subsequent entry biologic) is a biologic medical product that is almost an identical copy of an original product that is manufactured by a different company. Biosimilars are officially approved versions of original "innovator" products and can be manufactured when the original product's patent expires. Reference to the innovator product is an integral component of the approval. Unlike with generic drugs of the more common small-molecule type, biologics generally exhibit high molecular complexity and may be quite sensitive to changes in manufacturing processes. Despite that heterogeneity, all biopharmaceuticals, including biosimilars, must maintain consistent quality and clinical performance throughout their lifecycle. Drug-related authorities such as the European Medicines Agency (EMA) of the European Union, the United States Food and Drug Administration (FDA), and the Health Products and Food Branch of Health Canada hold the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supreme Court Of Canada
The Supreme Court of Canada (SCC; french: Cour suprême du Canada, CSC) is the Supreme court, highest court in the Court system of Canada, judicial system of Canada. It comprises List of Justices of the Supreme Court of Canada, nine justices, whose decisions are the ultimate application of Canadian law, and grants permission to between 40 and 75 litigants each year to appeal decisions rendered by provincial, territorial and federal Appeal, appellate courts. The Supreme Court is bijural, hearing cases from two major legal traditions (common law and Civil law (legal system), civil law) and bilingual, hearing cases in both Official bilingualism in Canada, official languages of Canada (English language, English and French language, French). The effects of any judicial decision on the common law, on the interpretation of statutes, or on any other application of law, can, in effect, be nullified by legislation, unless the particular decision of the court in question involves applicatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bristol-Myers Squibb
The Bristol Myers Squibb Company (BMS) is an American multinational pharmaceutical company. Headquartered in New York City, BMS is one of the world's largest pharmaceutical companies and consistently ranks on the ''Fortune'' 500 list of the largest U.S. corporations. For fiscal 2021, it had a total revenue of $46.4 billion. Bristol Myers Squibb manufactures prescription pharmaceuticals and biologics in several therapeutic areas, including cancer, HIV/AIDS, cardiovascular disease, diabetes, hepatitis, rheumatoid arthritis, and psychiatric disorders. BMS's primary research and development (R&D) sites are located in Lawrence, New Jersey (formerly Squibb, near Princeton), Summit, New Jersey, formerly HQ of Celgene, New Brunswick, New Jersey, Redwood City, California, and Seville in Spain, with other sites in Devens and Cambridge, Massachusetts, East Syracuse, New York, Braine-l'Alleud, Belgium, Tokyo, Japan, Bangalore, India, and Wirral, United Kingdom. BMS previously had ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
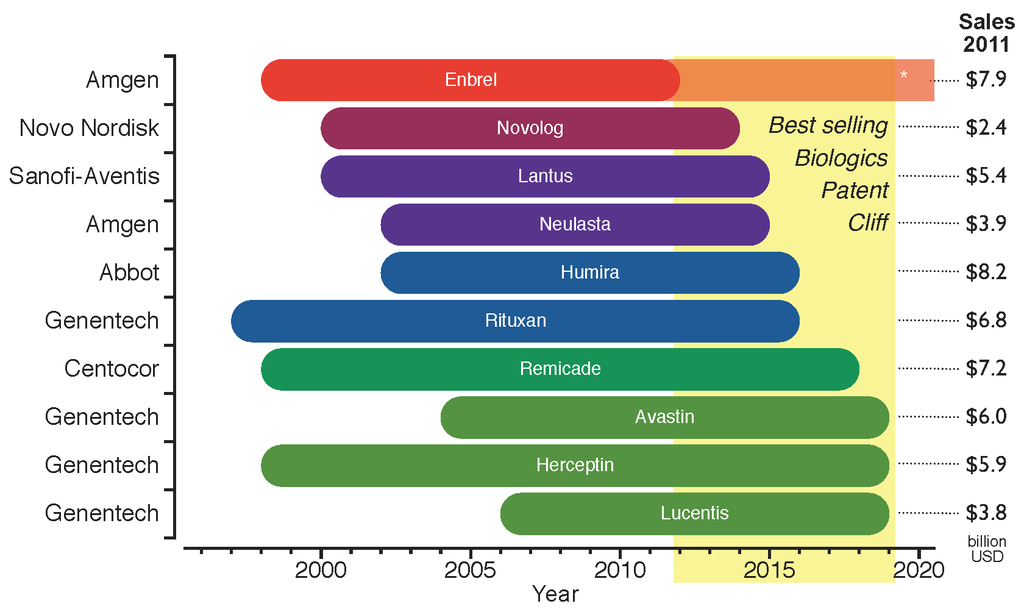
Neulasta
Pegfilgrastim, sold under the brand name Neulasta among others, is a PEGylated form of the recombinant human granulocyte colony-stimulating factor (GCSF) analog filgrastim. It serves to stimulate the production of white blood cells (neutrophils). Pegfilgrastim was developed by Amgen. Pegfilgrastim treatment can be used to stimulate bone marrow to produce more neutrophils to fight infection in patients undergoing chemotherapy. Pegfilgrastim has a human half-life of 15 to 80 hours, much longer than the parent filgrastim (3–4 hours). Pegfilgrastim was approved for medical use in the United States in January 2002, in the European Union in August 2002, and in Australia in September 2002. Medical uses Pegfilgrastim is indicated In medicine, an indication is a valid reason to use a certain test, medication, procedure, or surgery. There can be multiple indications to use a procedure or medication. An indication can commonly be confused with the term diagnosis. A diagnosis ... t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_(8493716336).jpg)

.jpg)