Fasciculin on:
[Wikipedia]
[Google]
[Amazon]
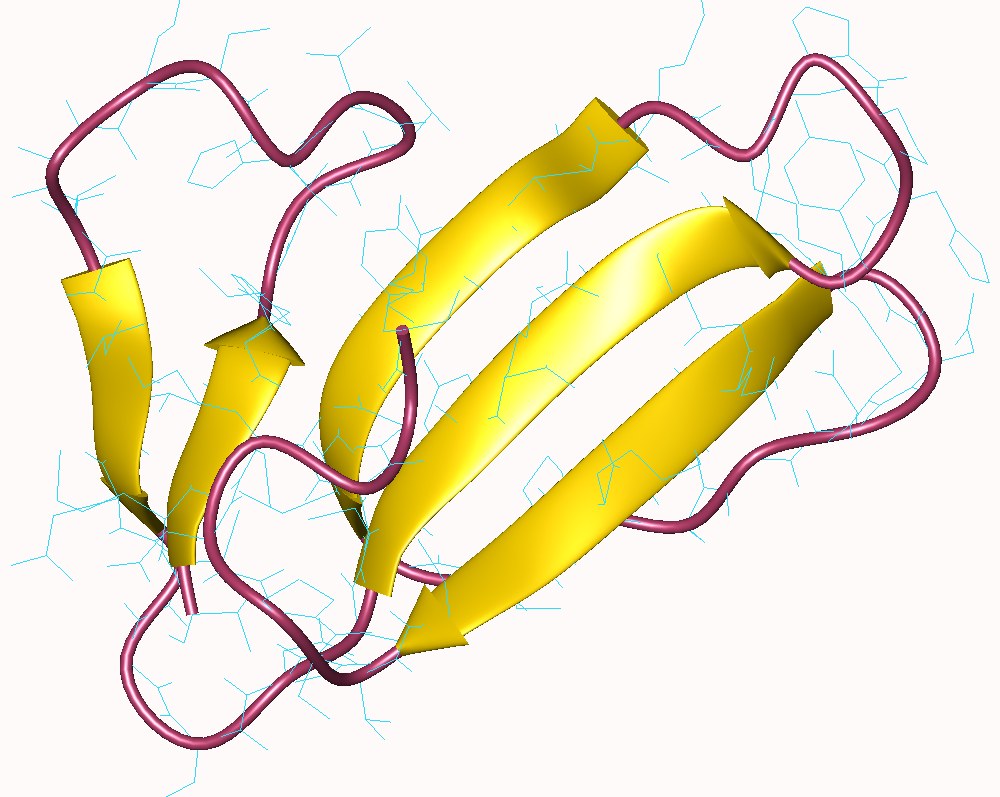 Fasciculins are a class of toxic proteins found in certain snake venoms, notably some species of
Fasciculins are a class of toxic proteins found in certain snake venoms, notably some species of
 Fasciculins are a class of toxic proteins found in certain snake venoms, notably some species of
Fasciculins are a class of toxic proteins found in certain snake venoms, notably some species of mamba
Mambas are fast moving highly venomous snakes of the genus ''Dendroaspis'' (which literally means "tree asp") in the family Elapidae. Four extant species are recognised currently; three of those four species are essentially arboreal and green ...
. Investigations have revealed distinct forms in some green mamba venoms, in particular FAS1 and FAS2 Fasciculins are so called because they cause intense fasciculation in muscle fascicle
A muscle fascicle is a bundle of skeletal muscle fibers surrounded by perimysium, a type of connective tissue.
Structure
Muscle cells are grouped into muscle fascicles by enveloping perimysium connective tissue. Fascicles are bundled togethe ...
s of susceptible organisms, such as the preferred prey of the snakes. This effect helps to incapacitate the muscles, either killing the prey, or paralysing it so that the snake can swallow it.
Fundamental mechanism of action
The mechanism of action of the FAS proteins is associated with their ability to attach to molecules of the acetylcholinesterase in the muscles and their neuromuscular junctions, and thereby interfere with their necessary neuromodulatory inhibition.Molecular nature and physiological effect
Fasciculins from mambas inhibit mammalian and fish acetylcholinesterases intensely, but are less active against the correspondingenzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
s in insects, reptiles and birds. As one might expect of fast-acting venoms, they are fairly small proteins of about 61 amino acid residues. Their three-dimensional shape is three-fingered, and is secured by four cross-linking disulfide bridge
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
s.
Disruption of acetylcholine signalling
Venom disrupters of acetylcholineneurotransmission
Neurotransmission (Latin: ''transmissio'' "passage, crossing" from ''transmittere'' "send, let through") is the process by which signaling molecules called neurotransmitters are released by the axon terminal of a neuron (the presynaptic neuron), ...
generally penetrate the neuromuscular junction, where they interfere with either the production or reception of acetylcholine, or the hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolys ...
of acetylcholine after it has achieved its function of neurotransmission; mamba fasciculins prevent the final stage of this process by binding to acetylcholinesterase and blocking its action on acetylcholine; the result is that after the acetylcholine has transmitted the required stimulus, it continues with the stimulus after it has become inappropriate. That mechanism is in some ways similar to the effect of the so-called organophosphate nerve agent
Nerve agents, sometimes also called nerve gases, are a class of organic chemicals that disrupt the mechanisms by which nerves transfer messages to organs. The disruption is caused by the blocking of acetylcholinesterase (AChE), an enzyme that ...
s; the blockage of the acetylcholinesterase action is what causes the fasciculation that inspired the name ''fasciculin''.
Docking and activity
In mammalian acetylcholinesterase two conserved peripheral anionic residues form part of the enzyme where the FAS molecule docks. Insect and avian acetylcholinesterases lack the two residues in those positions, and that drastically reduces their affinity for mamba fasciculins. However, there is a significant, though reduced, toxic effect, because several basic residues in the venom protein still establish and maintain contacts with the enzyme. This is unusual in protein complementarity, in that it involves attractions between multiple charged residues, but without any salt linkage between the molecules.References
{{Acetylcholine metabolism and transport modulators Acetylcholinesterase inhibitors Neurotoxins Vertebrate toxins