Elexacaftor/ivacaftor/tezacaftor on:
[Wikipedia]
[Google]
[Amazon]
Elexacaftor/tezacaftor/ivacaftor, sold under the brand names Trikafta (US) and Kaftrio (EU), is a
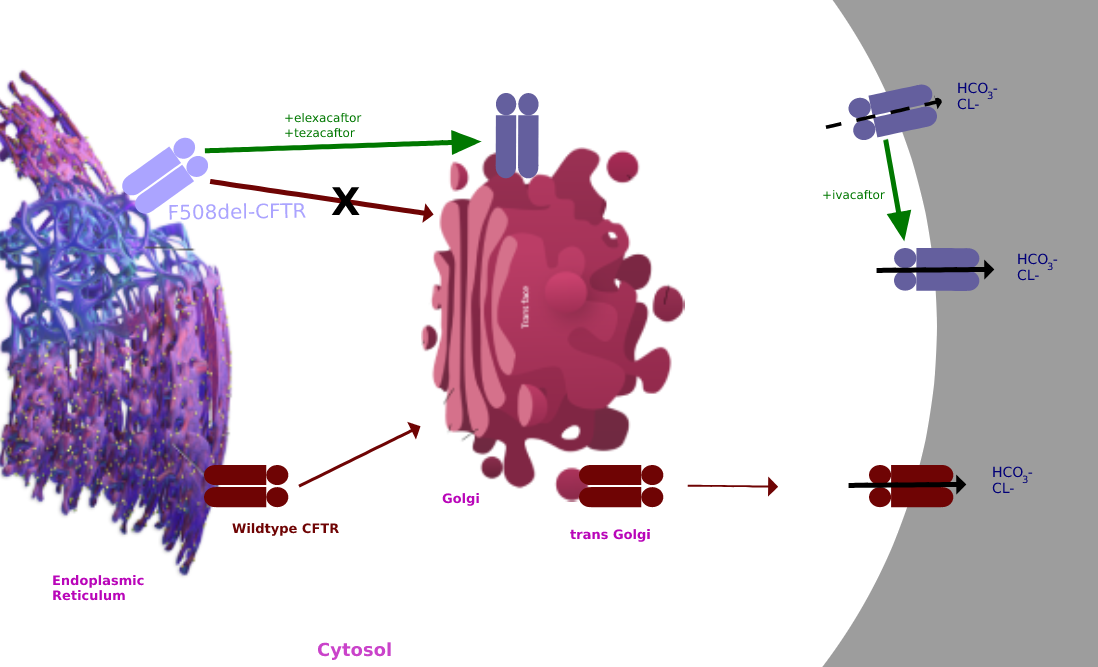



 Elexacaftor and Tezacaftor act as CFTR correctors to repair F508del processing by binding to the CFTR protein to increase the availability of CFTR protein on the cell surface. They work by modulating the position of the CFTR protein into the right position on the cell surface.
The combination of increased CFTR protein in the correct position on the cell surface with ivacaftor's potentiation of chloride channel opening results in increased transport of chloride and thinned mucus secretions.
Elexacaftor and Tezacaftor act as CFTR correctors to repair F508del processing by binding to the CFTR protein to increase the availability of CFTR protein on the cell surface. They work by modulating the position of the CFTR protein into the right position on the cell surface.
The combination of increased CFTR protein in the correct position on the cell surface with ivacaftor's potentiation of chloride channel opening results in increased transport of chloride and thinned mucus secretions.

fixed-dose combination
A combination drug or a fixed-dose combination (FDC) is a medicine that includes two or more active ingredients combined in a single dosage form. Terms like "combination drug" or "combination drug product" can be common shorthand for a FDC pr ...
medication used to treat cystic fibrosis. Elexacaftor/tezacaftor/ivacaftor is composed of a combination of ivacaftor
Ivacaftor is a medication used to treat cystic fibrosis in people with certain mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (primarily the G551D mutation), who account for 4–5% cases of cystic fibrosis. It ...
, a chloride channel opener, and elexacaftor and tezacaftor, CFTR modulators.
It is approved for use in the United States for people aged six years and older who have cystic fibrosis with a F508del mutation or other mutations in the CFTR gene. It is also approved for use in Canada, the European Union and Australia.
Medical uses
The combination isindicated
In medicine, an indication is a valid reason to use a certain test, medication, procedure, or surgery. There can be multiple indications to use a procedure or medication. An indication can commonly be confused with the term diagnosis. A diagnosis ...
for the treatment of people aged six years and older who have cystic fibrosis with a F508del mutation or other mutations in the CFTR gene.
Side effects
The most common side effects affecting more than 5% of patients are headache, upper respiratory tract infection, abdominal pain,diarrhea
Diarrhea, also spelled diarrhoea, is the condition of having at least three loose, liquid, or watery bowel movements each day. It often lasts for a few days and can result in dehydration due to fluid loss. Signs of dehydration often begin w ...
, rash
A rash is a change of the human skin which affects its color, appearance, or texture.
A rash may be localized in one part of the body, or affect all the skin. Rashes may cause the skin to change color, itch, become warm, bumpy, chapped, dry, c ...
, alanine aminotransferase
Alanine transaminase (ALT) is a transaminase enzyme (). It is also called alanine aminotransferase (ALT or ALAT) and was formerly called serum glutamate-pyruvate transaminase or serum glutamic-pyruvic transaminase (SGPT) and was first character ...
increase, nasal congestion
Nasal congestion is the blockage of nasal breathing usually due to membranes lining the nose becoming swollen from inflamed blood vessels.
Background
In about 85% of cases, nasal congestion leads to mouth breathing rather than nasal breathin ...
, blood creatine phosphokinase increase, aspartate aminotransferase increase, rhinorrhea
Rhinorrhea, rhinorrhoea, or informally runny nose is the free discharge of a thin mucus fluid from the nose; it is a common condition. It is a common symptom of allergies ( hay fever) or certain viral infections, such as the common cold or CO ...
, rhinitis
Rhinitis, also known as coryza, is irritation and inflammation of the mucous membrane inside the nose. Common symptoms are a stuffy nose, runny nose, sneezing, and post-nasal drip.
The inflammation is caused by viruses, bacteria, irrita ...
, influenza, sinusitis
Sinusitis, also known as rhinosinusitis, is inflammation of the mucous membranes that line the sinuses resulting in symptoms that may include thick nasal mucus, a plugged nose, and facial pain. Other signs and symptoms may include fever, head ...
and blood bilirubin increase.
Interactions
Concomitant use withCYP3A
Cytochrome P450, family 3, subfamily A, also known as CYP3A, is a human gene locus. A homologous locus is found in mice.
The CYP3A locus includes all the known members of the 3A subfamily of the cytochrome P450 superfamily of genes. These genes ...
inducers is not recommended. Dosage must be adjusted with moderate or strong CYP3A
Cytochrome P450, family 3, subfamily A, also known as CYP3A, is a human gene locus. A homologous locus is found in mice.
The CYP3A locus includes all the known members of the 3A subfamily of the cytochrome P450 superfamily of genes. These genes ...
inhibitors.
Other drugs with the potential for interaction include: warfarin
Warfarin, sold under the brand name Coumadin among others, is a medication that is used as an anticoagulant (blood thinner). It is commonly used to prevent blood clots such as deep vein thrombosis and pulmonary embolism, and to prevent st ...
, digoxin, statin
Statins, also known as HMG-CoA reductase inhibitors, are a class of lipid-lowering medications that reduce illness and mortality in those who are at high risk of cardiovascular disease. They are the most common cholesterol-lowering drugs.
Low ...
s, glyburide
Glibenclamide, also known as glyburide, is an antidiabetic medication used to treat type 2 diabetes. It is recommended that it be taken together with diet and exercise. It may be used with other antidiabetic medication. It is not recommended f ...
, nateglinide
Nateglinide (INN, trade name Starlix) is a drug for the treatment of type 2 diabetes. Nateglinide was developed by Ajinomoto, a Japanese company and sold by the Swiss pharmaceutical company Novartis.
Nateglinide belongs to the meglitinide class ...
, repaglinide
Repaglinide is an antidiabetic drug in the class of medications known as meglitinides, and was invented in 1983. Repaglinide is an oral medication used in addition to diet and exercise for blood sugar control in type 2 diabetes mellitus. The mec ...
.
Pharmacology
Cystic fibrosis and CFTR
Cystic fibrosis is an autosomal recessive genetic disorder of the CFTR protein which reduces chloride and sodium ion transport through the cell membrane, causing thicker than normal mucus secretions. The CFTR protein is found in epithelial cells of the lung, liver, pancreas, digestive tract, and reproductive tracts. CFTR has a role in the production of mucus, sweat, and digestive fluids. The thickened mucus can lead to inflammation, respiratory infections, and clogged ducts.Mechanism of action
Elexacaftor/tezacaftor/ivacaftor is a tridrug treatment in which the medications work together to increase the transport of chloride and sodium ions and correct fluid shifts that are dysregulated in cystic fibrosis.

CFTR channel potentiator
Ivacaftor
Ivacaftor is a medication used to treat cystic fibrosis in people with certain mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (primarily the G551D mutation), who account for 4–5% cases of cystic fibrosis. It ...
is a selective small-molecule potentiator of the CFTR
Cystic fibrosis transmembrane conductance regulator (CFTR) is a membrane protein and anion channel in vertebrates that is encoded by the ''CFTR'' gene.
Geneticist Lap-Chee Tsui and his team identified the CFTR gene in 1989 as the gene linked wi ...
protein that increases the protein's ability to open chloride channels. Its effectiveness is highly dependent on the amount of CFTR protein at the cell surface and the responsiveness of the mutant CFTR protein. Ivacaftor's primary target is to treat class III CFTR gating mutations like G551D as well as other less common mutations. In the crystalline figure, you can see ivacaftor, shown as a gray ball and stick model on the bottom-right, bound to CFTR docked in a cleft formed by transmembrane helices at the protein-lipid interface.
CFTR correctors

Pharmacokinetics
Elexacaftor/tezacaftor/ivacaftor is primarily metabolized by CYP3A4 /5. This medication should be taken with a high fat meal to improve absorption through the gut. It is excreted as metabolites or unchanged mainly through feces and to a smaller extent urine. The mean effective half-life of elexacaftor, tezacaftor, and ivacaftor is 27.4 hours, 25.1 hours, and 15 hours, respectively.History
A phase III trial showed people treated with elexacaftor/tezacaftor/ivacaftor improved inFEV1
Spirometry (meaning ''the measuring of breath'') is the most common of the pulmonary function tests (PFTs). It measures lung function, specifically the amount (volume) and/or speed (flow) of air that can be inhaled and exhaled. Spirometry is h ...
at four weeks with sustained improvement at 24 weeks. Rate of pulmonary exacerbation was 63% lower and sweat chloride concentration was 41.8 mmol/L lower. Its effectiveness is dependent on the type of CF mutations the patient has.
Society and culture
Legal status
United States
The combination was approved for use in the United States in 2019 for people twelve years and older with cystic fibrosis who have at least one F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which is estimated to represent 90% of the cystic fibrosis population. In December 2020, after an additional clinical trial was completed, and FDA approval was expanded for 177 other cystic fibrosis mutations. FDA approval for children aged 6–11 was added in January 2021, after a third clinical trial was completed. The U.S.Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is respon ...
(FDA) granted the application priority review
Priority review is a program of the United States Food and Drug Administration (FDA) to expedite the review process for drugs that are expected to have a particularly great impact on the treatment of a disease. The priority review voucher program ...
, in addition to fast track
The fast track is an informal English term meaning "the quickest and most direct route to achievement of a goal, as in competing for professional advancement". By definition, it implies that a less direct, slower route also exists.
Fast track or F ...
, breakthrough therapy
Breakthrough therapy is a United States Food and Drug Administration designation that expedites drug development that was created by Congress under Section 902 of the 9 July 2012 Food and Drug Administration Safety and Innovation Act. The FDA's "br ...
, and orphan drug designations. The drug's manufacturer Vertex Pharmaceuticals
Vertex Pharmaceuticals is an American biopharmaceutical company based in Boston, Massachusetts. It was one of the first biotech firms to use an explicit strategy of rational drug design rather than combinatorial chemistry. It maintains headqu ...
will receive a rare pediatric disease priority review voucher for having developed this therapy.
Australia
In March 2021, health regulators in Australia approved trikafta for patients aged 12 years and older with at least one copy of the F508del mutation. At the end of April 2022, it was placed on PBS, thus reducing the cost from tens of thousands of dollars a month, to tens of dollars a month.Canada
In June 2020, Health Canada approved Trikafta for patients ages 12 and up. In September 2021, the provincesAlberta
Alberta ( ) is one of the thirteen provinces and territories of Canada. It is part of Western Canada and is one of the three prairie provinces. Alberta is bordered by British Columbia to the west, Saskatchewan to the east, the Northwest Ter ...
and Saskatchewan
Saskatchewan ( ; ) is a province in western Canada, bordered on the west by Alberta, on the north by the Northwest Territories, on the east by Manitoba, to the northeast by Nunavut, and on the south by the U.S. states of Montana and North Dak ...
announced they will join Ontario
Ontario ( ; ) is one of the thirteen provinces and territories of Canada.Ontario is located in the geographic eastern half of Canada, but it has historically and politically been considered to be part of Central Canada. Located in Central C ...
in funding the medication. They will determine coverage on a case-by-case basis using criteria that has not yet been announced.
European Union
In June 2020, theCommittee for Medicinal Products for Human Use
The Committee for Medicinal Products for Human Use (CHMP), formerly known as Committee for Proprietary Medicinal Products (CPMP), is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regardin ...
(CHMP) of the European Medicines Agency (EMA) recommended its approval for the treatment of cystic fibrosis. It was approved for medical use in the European Union in August 2020.
Norway
On 25 April 2022, ''Beslutningsforum for nye metoder'' approved Kaftrio for treatment of cystic fibrosis.New Zealand
In February 2022, Pharmac recommended, with medium priority, funding for patients age 12 and above.Spain
In November 2021, the Spanish government approved the reimbursement of Kaftrio for patients ages 12 and older with at least one copy of the F508del mutation.Economics
United States
The list price of a year's treatment in the US is . However, a 2020 report byInstitute for Clinical and Economic Review The Institute for Clinical and Economic Review (ICER) is a Boston-based independent nonprofit organization that seeks to place a value on medical care by providing comprehensive clinical and cost-effectiveness analyses of treatments, tests, and proc ...
found that the price has made the treatment not cost effective and that "an appropriate health-benefit price would range from $67,900–$85,500 per year".
Australia
Following the listing of Trikafta on thePharmaceutical Benefits Scheme
The Pharmaceutical Benefits Scheme (PBS) is a program of the Australian Government that subsidises prescription medication for Australian citizens and permanent residents, as well as international visitors covered by a reciprocal health care ag ...
in 2022, the cost for CF patients 12 years or older who have at least one F508del mutation in the cystic fibrosis transmembrane conductance regulator gene is $42.50 per month, or $6.80 for concession card holders.
Research
CFTR mutations that are responsive to elexacaftor/tezacaftor/ivacaftor were determined by an in-vitro study of Fischer Rat Thyroid (FRT) cells that expressed mutant CFTR. Elexacaftor/tezacaftor/ivacaftor showed effectiveness with mutations where the CFTR protein was being successfully delivered to the cell surface.References
External links
* * {{Portal bar , Medicine Breakthrough therapy Combination drugs Cystic fibrosis Orphan drugs