|
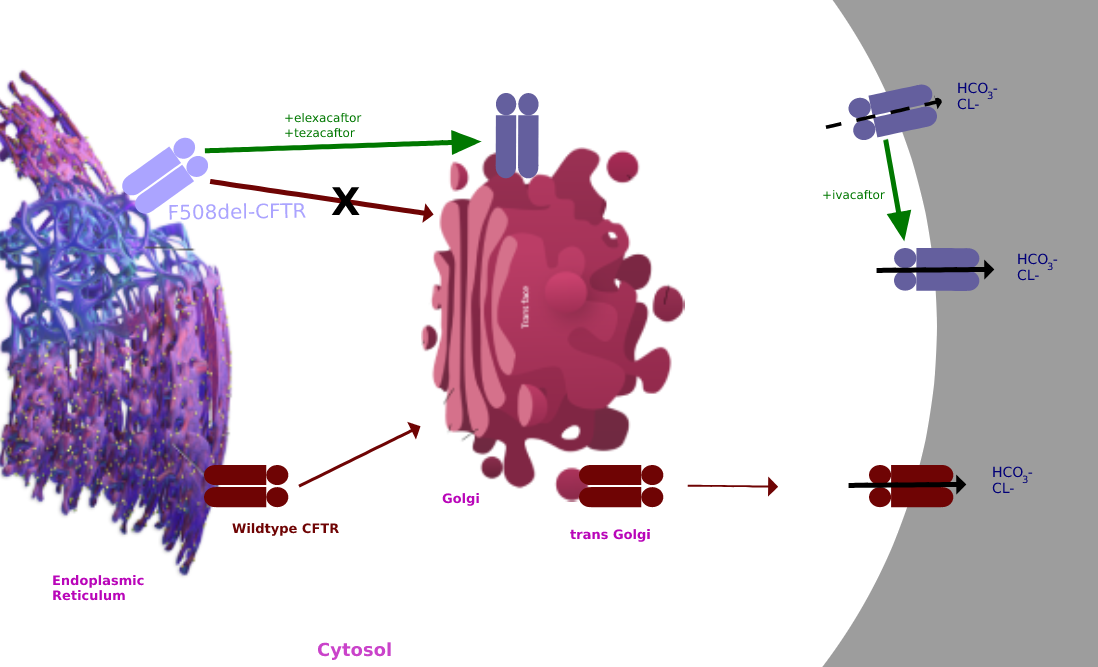
Tezacaftor
Tezacaftor is a drug used for the treatment of cystic fibrosis (CF) in people six years and older, who have a F508del mutation, the most common type of mutation in the CFTR gene. It is sold as a fixed-dose combination with ivacaftor under the brand name Symdeko. It was approved by the U.S. FDA in 2018. The combination of elexacaftor, tezacaftor, and ivacaftor is being sold as Trikafta. In 2019, the U.S. Food and Drug Administration (FDA) approved a combination of elexacaftor, tezacaftor, and ivacaftor. Mechanism of action Tezacaftor acts as a corrector to help the folding and presentation of the CFTR protein to the cell surface, which improves its function for individuals with a F508del mutation. Clinical trials The EVOLVE and EXPAND study findings were published in 2017. EVOLVE trial The EVOLVE trial analyzed tezacaftor/ivacaftor in patients with cystic fibrosis, specifically with the homozygous for Phe508del mutation. The EVOLVE trial is a phase 3, double-blinded, mu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trikafta
Elexacaftor/tezacaftor/ivacaftor, sold under the brand names Trikafta (US) and Kaftrio (EU), is a fixed-dose combination medication used to treat cystic fibrosis. Elexacaftor/tezacaftor/ivacaftor is composed of a combination of ivacaftor, a chloride channel opener, and elexacaftor and tezacaftor, CFTR modulators. It is approved for use in the United States for people aged six years and older who have cystic fibrosis with a F508del mutation or other mutations in the CFTR gene. It is also approved for use in Canada, the European Union and Australia. Medical uses The combination is indicated for the treatment of people aged six years and older who have cystic fibrosis with a F508del mutation or other mutations in the CFTR gene. Side effects The most common side effects affecting more than 5% of patients are headache, upper respiratory tract infection, abdominal pain, diarrhea, rash, alanine aminotransferase increase, nasal congestion, blood creatine phosphokinase increas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ivacaftor
Ivacaftor is a medication used to treat cystic fibrosis in people with certain mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (primarily the G551D mutation), who account for 4–5% cases of cystic fibrosis. It is also included in combination medications, lumacaftor/ivacaftor, tezacaftor/ivacaftor, and elexacaftor/tezacaftor/ivacaftor which are used to treat people with cystic fibrosis. Ivacaftor was developed by Vertex Pharmaceuticals in conjunction with the Cystic Fibrosis Foundation and is the first medication that treats the underlying cause rather than the symptoms of the disease. It was approved by the U.S. Food and Drug Administration (FDA) in January 2012. It is one of the most expensive drugs, costing over per year, which has led to criticism of the high cost. The combination drug lumacaftor/ivacaftor was approved by the FDA in July 2015. Cystic fibrosis is caused by any one of several defects in the CFTR protein, which regulates fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elexacaftor
Elexacaftor is a medication that acts as cystic fibrosis transmembrane conductance regulator (CFTR) corrector. It is available in a single pill with ivacaftor and tezacaftor; the fixed-dose combination, elexacaftor/tezacaftor/ivacaftor (brand name ''Trikafta''), is used to treat people with cystic fibrosis who are homozygous for the f508del mutation. This combination was approved for medical use in the United States in 2019. The fixed-dose combination elexacaftor/tezacaftor/ivacaftor (Kaftrio) was approved for medical use in the European Union The European Union (EU) is a supranational political and economic union of member states that are located primarily in Europe. The union has a total area of and an estimated total population of about 447million. The EU has often been de ... in August 2020, for the treatment of cystic fibrosis. References External links * Breakthrough therapy Cystic fibrosis Orphan drugs {{respiratory-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystic Fibrosis
Cystic fibrosis (CF) is a rare genetic disorder that affects mostly the lungs, but also the pancreas, liver, kidneys, and intestine. Long-term issues include difficulty breathing and coughing up mucus as a result of frequent lung infections. Other signs and symptoms may include sinus infections, poor growth, fatty stool, clubbing of the fingers and toes, and infertility in most males. Different people may have different degrees of symptoms. Cystic fibrosis is inherited in an autosomal recessive manner. It is caused by the presence of mutations in both copies of the gene for the cystic fibrosis transmembrane conductance regulator (CFTR) protein. Those with a single working copy are carriers and otherwise mostly healthy. CFTR is involved in the production of sweat, digestive fluids, and mucus. When the CFTR is not functional, secretions which are usually thin instead become thick. The condition is diagnosed by a sweat test and genetic testing. Screening of infants at birth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vertex Pharmaceuticals Incorporated
Vertex Pharmaceuticals is an American biopharmaceutical company based in Boston, Massachusetts. It was one of the first biotech firms to use an explicit strategy of rational drug design rather than combinatorial chemistry. It maintains headquarters in South Boston, Massachusetts, and three research facilities, in San Diego, California, and Milton Park, near Oxford, England. History Vertex was founded in 1989 by Joshua Boger and Kevin J. Kinsella . to ''"transform the way serious diseases are treated."'' The company's beginnings were profiled by Barry Werth in the 1994 book, '' The Billion-Dollar Molecule''. His 2014 book, ''The Antidote: Inside the World of New Pharma'', chronicled the company's subsequent development over the next two decades. By 2004, its product pipeline focused on viral infections, inflammatory and autoimmune disorders, and cancer. In 2009, the company had about 1,800 employees, including 1,200 in the Boston area. By 2019 there were about 2,500 employ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drug
An orphan drug is a pharmaceutical agent developed to treat medical conditions which, because they are so rare, would not be profitable to produce without government assistance. The conditions are referred to as orphan diseases. The assignment of orphan status to a disease and to drugs developed to treat it is a matter of public policy in many countries and has yielded medical breakthroughs that might not otherwise have been achieved, due to the economics of drug research and development. In the U.S. and the EU, it is easier to gain marketing approval for an orphan drug. There may be other financial incentives, such as an extended period of exclusivity, during which the producer has sole rights to market the drug. All are intended to encourage development of drugs which would otherwise lack sufficient profit motive to attract corporate research budgets and personnel. Definition According to the US Food and Drug Administration (FDA), an orphan drug is defined as one "intended for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indoles
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. The corresponding substituent is called indolyl. Indole undergoes electrophilic substitution, mainly at position 3 (see diagram i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropanes
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself is mainly of theoretical interest but many of its derivatives are of commercial or biological significance. History Cyclopropane was discovered in 1881 by August Freund, who also proposed the correct structure for the substance in his first paper. Freund treated 1,3-dibromopropane with sodium, causing an intramolecular Wurtz reaction leading directly to cyclopropane. The yield of the reaction was improved by Gustavson in 1887 with the use of zinc instead of sodium. Cyclopropane had no commercial application until Henderson and Lucas discovered its anaesthetic properties in 1929; industrial production had begun by 1936. In modern anaesthetic practice, it has been superseded by other agents. Anaesthesia Cyclopropane was introduced into clin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Priority Review
Priority review is a program of the United States Food and Drug Administration (FDA) to expedite the review process for drugs that are expected to have a particularly great impact on the treatment of a disease. The priority review voucher program is a program that grants a voucher for priority review to a drug developer as an incentive to develop treatments for disease indications with limited profitability. Priority review vouchers are currently earned by pharmaceutical companies for the development and approval of drugs treating neglected tropical diseases, rare pediatric diseases, and "medical countermeasures" for terrorism. The voucher can be used for future drugs that could have wider indications for use, but the company is required to pay a fee (approximately $2.8 million) to use the voucher. When seeking approval for a drug, manufacturers can apply to the FDA for priority review. This is granted when a drug is intended to treat a serious condition and would "provide a sig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
New England Journal Of Medicine
''The New England Journal of Medicine'' (''NEJM'') is a weekly medical journal published by the Massachusetts Medical Society. It is among the most prestigious peer-reviewed medical journals as well as the oldest continuously published one. History In September 1811, John Collins Warren, a Boston physician, along with James Jackson, submitted a formal prospectus to establish the ''New England Journal of Medicine and Surgery and Collateral Branches of Science'' as a medical and philosophical journal. Subsequently, the first issue of the ''New England Journal of Medicine and Surgery and the Collateral Branches of Medical Science'' was published in January 1812. The journal was published quarterly. In 1823, another publication, the ''Boston Medical Intelligencer'', appeared under the editorship of Jerome V. C. Smith. The editors of the ''New England Journal of Medicine and Surgery and the Collateral Branches of Medical Science'' purchased the weekly ''Intelligencer'' for $600 in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Placebo
A placebo ( ) is a substance or treatment which is designed to have no therapeutic value. Common placebos include inert tablets (like sugar pills), inert injections (like saline), sham surgery, and other procedures. In general, placebos can affect how patients perceive their condition and encourage the body's chemical processes for relieving pain and a few other symptoms, but have no impact on the disease itself. Improvements that patients experience after being treated with a placebo can also be due to unrelated factors, such as regression to the mean (a statistical effect where an unusually high or low measurement is likely to be followed by a less extreme one). The use of placebos in clinical medicine raises ethical concerns, especially if they are disguised as an active treatment, as this introduces dishonesty into the doctor–patient relationship and bypasses informed consent. While it was once assumed that this deception was necessary for placebos to have any effect, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |