Chromium(III) boride on:
[Wikipedia]
[Google]
[Amazon]
Chromium(III) boride, also known as chromium monoboride (CrB), is an 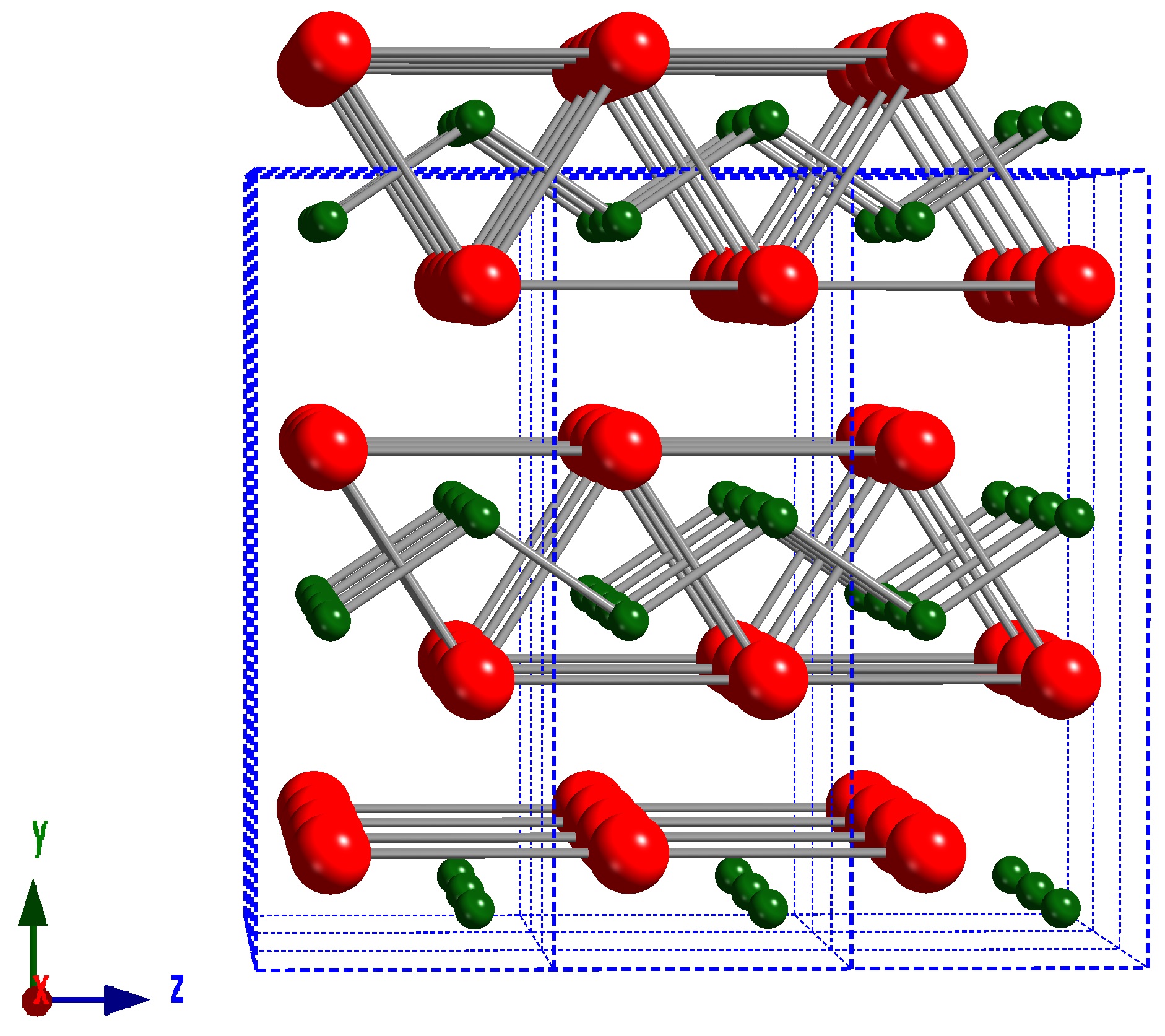
inorganic compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemist ...
with the chemical formula CrB. It is one of the six stable binary borides of chromium, which also include Cr2B, Cr5B3, Cr3B4, CrB2, and CrB4. Like many other transition metal borides, it is extremely hard (21-23 GPa), has high strength (690 MPa bending strength
Flexural strength, also known as modulus of rupture, or bend strength, or transverse rupture strength is a material property, defined as the stress in a material just before it yields in a flexure test. The transverse bending test is most freque ...
), conducts heat and electricity as well as many metallic alloys, and has a high melting point (~2100 °C). Unlike pure chromium, CrB is known to be a paramagnetic, with a magnetic susceptibility
In electromagnetism, the magnetic susceptibility (Latin: , "receptive"; denoted ) is a measure of how much a material will become magnetized in an applied magnetic field. It is the ratio of magnetization (magnetic moment per unit volume) to the ap ...
that is only weakly dependent on temperature. Due to these properties, among others, CrB has been considered as a candidate material for wear resistant coatings and high-temperature diffusion barrier A diffusion barrier is a thin layer (usually micrometres thick) of metal usually placed between two other metals. It is done to act as a barrier to protect either one of the metals from corrupting the other..
Adhesion of a plated metal layer to it ...
s.
It can be synthesized as powders by many methods including direct reaction of the constituent elemental powders, self-propagating high-temperature synthesis (SHS), borothermic reduction, and molten salt growth. Slow-cooling of molten aluminum solutions from high-temperatures has been used to grow large single crystals, with a maximum size of 0.6 mm x 0.6 mm x 8.3 mm.
CrB has an orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a r ...
crystal structure ( space group ''Cmcm'') that was first discovered in 1951, and subsequently confirmed by later work using single crystals. The crystal structure can be visualized as slabs face-sharing BCr6 trigonal prisms, in the ac-plane, that are stacked parallel to the ''<010>'' crystallographic direction. Similar to Cr3B4 and Cr2B3, the B atoms in the structure form covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
s with each other and are characterized by unidirectional B-B- chains parallel to the ''<001>'' crystallographic direction. The transition metal monoborides VB, NbB, TaB, and NiB have the same crystal structure.

References
Chromium(III) compounds Borides {{Inorganic-compound-stub