Chemical structure diagram on:
[Wikipedia]
[Google]
[Amazon]
 The structural formula of a
The structural formula of a



 Chirality in skeletal formulas is indicated by the
Chirality in skeletal formulas is indicated by the
 Wavy single bonds represent unknown or unspecified stereochemistry or a mixture of isomers. For example, the above diagram shows the fructose molecule with a wavy bond to the HOCH2- group at the left. In this case the two possible ring structures are in chemical equilibrium with each other and also with the open-chain structure. The ring automatically opens and closes, sometimes closing with one stereochemistry and sometimes with the other.
Skeletal formulas can depict ''cis'' and ''trans'' isomers of alkenes. Wavy single bonds are the standard way to represent unknown or unspecified stereochemistry or a mixture of isomers (as with tetrahedral stereocenters). A crossed double-bond has been used sometimes, but is no longer considered an acceptable style for general use.
Wavy single bonds represent unknown or unspecified stereochemistry or a mixture of isomers. For example, the above diagram shows the fructose molecule with a wavy bond to the HOCH2- group at the left. In this case the two possible ring structures are in chemical equilibrium with each other and also with the open-chain structure. The ring automatically opens and closes, sometimes closing with one stereochemistry and sometimes with the other.
Skeletal formulas can depict ''cis'' and ''trans'' isomers of alkenes. Wavy single bonds are the standard way to represent unknown or unspecified stereochemistry or a mixture of isomers (as with tetrahedral stereocenters). A crossed double-bond has been used sometimes, but is no longer considered an acceptable style for general use.

File:Water with 4 single electrons.svg, The
CH3CH2OH ((CH3)2CHOH or CH(CH3)2OH (C=O
is implied through the O being placed in the brackets. For example:
CH3C(O)CH3 (CHO , Carboxylic acids as CO2H or COOH , CO2R or COOR . However, the use of condensed formulas does not give an immediate idea of the molecular geometry of the compound or the number of bonds between the carbons, it needs to be recognized based on the number of atoms attached to the carbons and if there are any charges on the carbon.
Image:Isobutanol.svg, Skeletal formula of
Image:Newman projection butane -sc.svg, Newman projection of butane
Image:Sawhorse projection butane -sc.svg, Sawhorse projection of butane


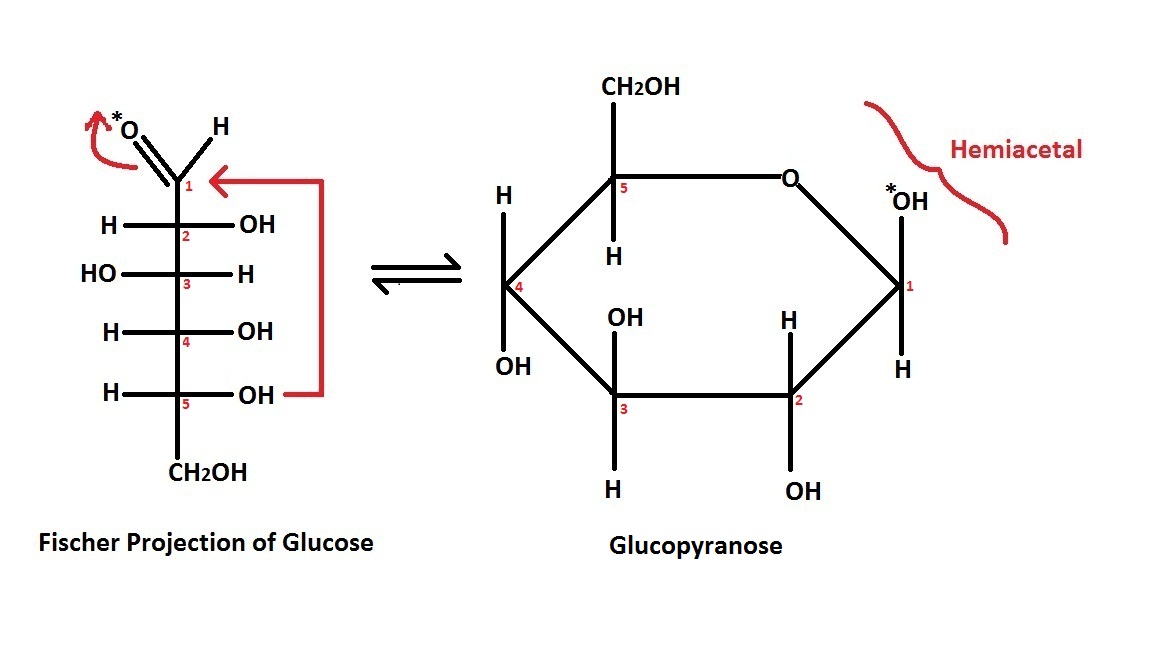
Image:beta-D-glucose Haworth formula.svg, Haworth projection of beta-D-Glucose
Image:DGlucose Fischer.svg, Fischer projection of D-Glucose
The Importance of Structural Formulas
*
{{DEFAULTSORT:Structural Formula Chemical formulas Chemical structures
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are possibly arranged in the real three-dimensional space
Space is the boundless three-dimensional extent in which objects and events have relative position and direction. In classical physics, physical space is often conceived in three linear dimensions, although modern physicists usually cons ...
. The chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
ing within the molecule is also shown, either explicitly or implicitly. Unlike other chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbol ...
types, which have a limited number of symbols and are capable of only limited descriptive power, structural formulas provide a more complete geometric representation of the molecular structure. For example, many chemical compounds exist in different isomeric forms, which have different enantiomeric structures but the same molecular formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
. There are multiple types of ways to draw these structural formulas such as: Lewis Structures
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons tha ...
, condensed formulas, skeletal formula
The skeletal formula, or line-angle formula or shorthand formula, of an organic compound is a type of molecular structural formula that serves as a shorthand representation of a molecule's bonding and some details of its molecular geometry. A ...
s, Newman projection
A Newman projection is a drawing that helps visualize the 3-dimensional structure of a molecule. This projection most commonly sights down a carbon-carbon bond, making it a very useful way to visualize the stereochemistry of alkanes. A Newman pro ...
s, Cyclohexane conformation
In organic chemistry, cyclohexane conformations are any of several three-dimensional shapes adopted by molecules of cyclohexane. Because many compounds feature structurally similar six-membered rings, the structure and dynamics of cyclohexane ...
s, Haworth projection
In chemistry, a Haworth projection is a common way of writing a structural formula to represent the cyclic structure of monosaccharides with a simple three-dimensional perspective. Haworth projection approximate the shapes of the actual mole ...
s, and Fischer projection
In chemistry, the Fischer projection, devised by Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates ...
s.
Several systematic chemical naming formats, as in chemical database
A chemical database is a database specifically designed to store chemical information. This information is about chemical and crystal structures, spectra, reactions and syntheses, and thermophysical data.
Types of chemical databases
Bioactivi ...
s, are used that are equivalent to, and as powerful as, geometric structures. These chemical nomenclature systems include SMILES
The simplified molecular-input line-entry system (SMILES) is a specification in the form of a line notation for describing the structure of chemical species using short ASCII strings. SMILES strings can be imported by most molecule editors f ...
, InChI
The International Chemical Identifier (InChI or ) is a textual identifier for chemical substances, designed to provide a standard way to encode molecular information and to facilitate the search for such information in databases and on the we ...
and CML. These systematic chemical names can be converted to structural formulas and vice versa, but chemists nearly always describe a chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
or synthesis using structural formulas rather than chemical names, because the structural formulas allow the chemist to visualize the molecules and the structural changes that occur in them during chemical reactions. ChemSketch
ACD/ChemSketch is a molecular modeling program used to create and modify images of chemical structures. Also, there is a software that allows molecules and molecular models displayed in two and three dimensions, to understand the structure of c ...
and ChemDraw
ChemDraw is a molecule editor first developed in 1985 by David A. Evans and Stewart Rubenstein (later by the cheminformatics company CambridgeSoft). The company was sold to PerkinElmer in the year 2011. ChemDraw, along with Chem3D and ChemFinde ...
are popular downloads/websites that allow users to draw reactions and structural formulas, typically in the Lewis Structure style.
Structures in Structural Formulas
Bonds
Bonds are often shown as a line that connects one atom to another. One line indicates a single bond. Two lines indicate adouble bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betwee ...
, and three lines indicate a triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order o ...
. In some structures the atoms in between each bond are specified and shown. However, in some structures, the carbon molecules are not written out specifically. Instead, these carbons are indicated by a corner that forms when two lines connect. Additionally, Hydrogen atoms are implied and not usually drawn out. These can be inferred based on how many other atoms the carbon is attached to. For example, if Carbon A is attached to one other Carbon B, Carbon A will have three hydrogens in order to fill its octet.


Electrons

Electrons
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
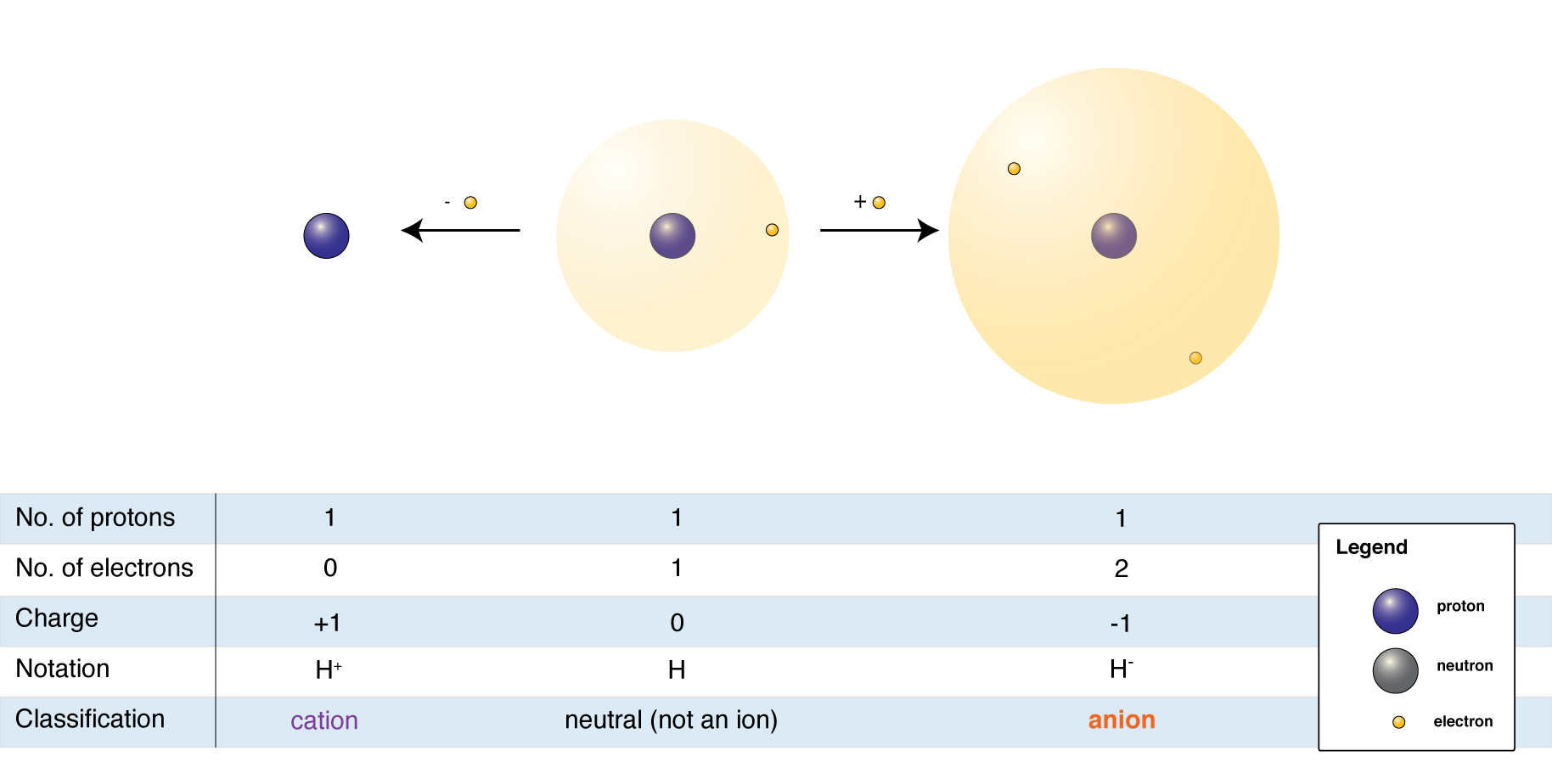
are usually shown as colored in circles. One circle indicates one electron. Two circles indicate a pair of electrons. Typically, a pair of electrons will also indicate a negative charge. By using the colored circles, the number of electrons in the valence shell of each respective atom is indicated providing further descriptive information regarding the reactive capacity of that atom in the molecule.
Charges
Often times, atoms will have a positive or negativecharge
Charge or charged may refer to:
Arts, entertainment, and media Films
* '' Charge, Zero Emissions/Maximum Speed'', a 2011 documentary
Music
* ''Charge'' (David Ford album)
* ''Charge'' (Machel Montano album)
* ''Charge!!'', an album by The Aqu ...
as their octet may not be complete. If the atom is missing a pair of electrons or has a proton, it will have a positive charge. If the atom has electrons that are not bonded to another atom, there will be a negative charge. In structural formulas, the positive charge is indicated by ⊕ , and the negative charge is indicated by ⊖ .

Stereochemistry (Skeletal Formula)
Natta projection
In chemistry, the Natta projection (named for Italian chemist Giulio Natta) is a way to depict molecules with complete stereochemistry in two dimensions in a skeletal formula. In a hydrocarbon molecule with all carbon atoms making up the backb ...
method. Stereochemistry is used to show the relative spatial arrangement of atoms in a molecule. Wedges are used to show this, and there are two types: dashed and filled. A filled wedge indicates that the atom is in the front of the molecule; it is pointing above the plane of the paper towards the front. A dashed wedge indicates that the atom is behind the molecule; it is pointing below the plane of the paper. When a straight, un-dashed line is used, the atom is in the plane of the paper. This spatial arrangement provides an idea of the molecule in a 3-dimensional space and there are constraints as to how the spatial arrangements can be arranged.
Unspecified Stereochemistry
Lewis structures
Lewis structure
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons t ...
s (or "Lewis dot structures") are flat graphical formulas that show atom connectivity and lone pair or unpaired electrons, but not three-dimensional structure. This notation is mostly used for small molecules. Each line represents the two electrons of a single bond. Two or three parallel lines between pairs of atoms represent double or triple bonds, respectively. Alternatively, pairs of dots may be used to represent bonding pairs. In addition, all non-bonded electrons (paired or unpaired) and any formal charges
In chemistry, a formal charge (F.C. or q), in the covalent view of chemical bonding, is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electroneg ...
on atoms are indicated. Through the use of Lewis structure
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons t ...
s, the placement of electrons, whether it is in a bond or in lone pairs, will allow for the identification of the formal charges of the atoms in the molecule to understand the stability and determine the most likely molecule (based on molecular geometry
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that dete ...
difference) that would be formed in a reaction. Lewis structure
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons t ...
s do give some thought to the geometry of the molecule as often times, the bonds are drawn at certain angles to represent the molecule in real life. Lewis structure
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons t ...
is best used to calculate formal charges or how atoms bond to each other as both electrons and bonds are shown. Lewis structure
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons t ...
s give an idea of the molecular and electronic geometry which varies based on the presence of bonds and lone pairs and through this one could determine the bond angles
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that deter ...
and hybridization as well.
Lewis structure
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons t ...
of water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
Condensed formulas
In early organic-chemistry publications, where use of graphics was strongly limited, a typographic system arose to describe organic structures in a line of text. Although this system tends to be problematic in application to cyclic compounds, it remains a convenient way to represent simple structures: :ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
)
Parentheses are used to indicate multiple identical groups, indicating attachment to the nearest non-hydrogen atom on the left when appearing within a formula, or to the atom on the right when appearing at the start of a formula:
: 2-propanol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the sim ...
)
In all cases, all atoms are shown, including hydrogen atoms. It is also helpful to show the carbonyls where the
acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscib ...
)
Therefore, it is important to look to the left of the atom in the bracket to make sure what atom it is attached to. This is helpful when converting from condensed formula to another form of structural formula such as skeletal formula
The skeletal formula, or line-angle formula or shorthand formula, of an organic compound is a type of molecular structural formula that serves as a shorthand representation of a molecule's bonding and some details of its molecular geometry. A ...
or Lewis structure
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons t ...
s. There are different ways to show the various functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the re ...
s in the condensed formulas such as aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
as Ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides a ...
s as Skeletal formulas
Skeletal formula
The skeletal formula, or line-angle formula or shorthand formula, of an organic compound is a type of molecular structural formula that serves as a shorthand representation of a molecule's bonding and some details of its molecular geometry. A ...
s are the standard notation for more complex organic molecules. In this type of diagram, first used by the organic chemist Friedrich August Kekulé von Stradonitz, the carbon atoms are implied to be located at the vertices (corners) and ends of line segments rather than being indicated with the atomic symbol C. Hydrogen atoms attached to carbon atoms are not indicated: each carbon atom is understood to be associated with enough hydrogen atoms to give the carbon atom four bonds. The presence of a positive or negative charge
Charge or charged may refer to:
Arts, entertainment, and media Films
* '' Charge, Zero Emissions/Maximum Speed'', a 2011 documentary
Music
* ''Charge'' (David Ford album)
* ''Charge'' (Machel Montano album)
* ''Charge!!'', an album by The Aqu ...
at a carbon atom takes the place of one of the implied hydrogen atoms. Hydrogen atoms attached to atoms other than carbon must be written explicitly. An additional feature of skeletal formulas is that by adding certain structures the stereochemistry, that is the three-dimensional structure, of the compound can be determined. Often times, the skeletal formula can indicate stereochemistry through the use of wedges instead of lines. Solid wedges represent bonds pointing above the plane of the paper, whereas dashed wedges represent bonds pointing below the plane.
isobutanol
Isobutanol (IUPAC nomenclature: 2-methylpropan-1-ol) is an organic compound with the formula (CH3)2CHCH2OH (sometimes represented as ''i''-BuOH). This colorless, flammable liquid with a characteristic smell is mainly used as a solvent either dire ...
, (CH3)2CHCH2OHPerspective drawings
Newman projection and sawhorse projection
TheNewman projection
A Newman projection is a drawing that helps visualize the 3-dimensional structure of a molecule. This projection most commonly sights down a carbon-carbon bond, making it a very useful way to visualize the stereochemistry of alkanes. A Newman pro ...
and the sawhorse
In woodworking, a saw-horse or sawhorse (saw-buck, trestle, buck) is a trestle structure used to support a board or plank for sawing. A pair of sawhorses can support a plank, forming a scaffold. In certain circles, it is also known as a '' ...
projection are used to depict specific conformers
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds (refer to figure on single bond rotation). While any two arrangements of atoms in a molec ...
or to distinguish vicinal stereochemistry. In both cases, two specific carbon atoms and their connecting bond are the center of attention. The only difference is a slightly different perspective: the Newman projection looking straight down the bond of interest, the sawhorse projection looking at the same bond but from a somewhat oblique
Oblique may refer to:
* an alternative name for the character usually called a slash (punctuation) ( / )
*Oblique angle, in geometry
*Oblique triangle, in geometry
* Oblique lattice, in geometry
* Oblique leaf base, a characteristic shape of the b ...
vantage point. In the Newman projection, a circle is used to represent a plane perpendicular to the bond, distinguishing the substituents on the front carbon from the substituents on the back carbon. In the sawhorse projection, the front carbon is usually on the left and is always slightly lower. Sometimes, an arrow is used to indicate the front carbon. The sawhorse projection is very similar to a skeletal formula, and it can even use wedges instead of lines to indicate the stereochemistry of the molecule. The sawhorse projection is set apart from the skeletal formulas because the sawhorse projection is not a very good indicator of molecule geometry and molecular arrangement. Both a Newman and Sawhorse Projection can be used to create a Fischer Projection.
Cyclohexane conformations
Certain conformations of cyclohexane and other small-ring compounds can be shown using a standard convention. For example, the standardchair conformation
In organic chemistry, cyclohexane conformations are any of several three-dimensional shapes adopted by molecules of cyclohexane. Because many compounds feature structurally similar six-membered rings, the structure and dynamics of cyclohexane are ...
of cyclohexane involves a perspective view from slightly above the average plane of the carbon atoms and indicates clearly which groups are axial (pointing vertically up or down) and which are equatorial Equatorial may refer to something related to:
*Earth's equator
**the tropics, the Earth's equatorial region
**tropical climate
*the Celestial equator
** equatorial orbit
**equatorial coordinate system
** equatorial mount, of telescopes
* equatorial ...
(almost horizontal, slightly slanted up or down). Bonds in front may or may not be highlighted with stronger lines or wedges. The conformations progress as follows: chair to half-chair to twist-boat to boat to twist-boat to half-chair to chair. The cyclohexane conformations may also be used to show the potential energy present at each stage as shown in the diagram. The chair conformations (A) have the lowest energy, whereas the half-chair conformations (D) have the highest energy. There is a peak/local maximum at the boat conformation (C), and there are valleys/local minimums at the twist-boat conformations (B). In addition, cyclohexane conformations can be used to indicate if the molecule has any 1,3 diaxial-interactions which are steric interactions between axial substituents on the 1,3, and 5 carbons.Haworth projection
TheHaworth projection
In chemistry, a Haworth projection is a common way of writing a structural formula to represent the cyclic structure of monosaccharides with a simple three-dimensional perspective. Haworth projection approximate the shapes of the actual mole ...
is used for cyclic sugars. Axial and equatorial positions are not distinguished; instead, substituents are positioned directly above or below the ring atom to which they are connected. Hydrogen substituents are typically omitted.
However, an important thing to keep in mind while reading an Haworth projection is that the ring structures are not flat. Therefore, Haworth does not provide 3-D shape. Sir Norman Haworth, was a British Chemist, who won a Noble Prize for his work on Carbohydrates and discovering the structure of Vitamin C. During his discovery, he also deducted different structural formulas which are now referred to as Haworth Projections. In a Haworth Projection a pyranose sugar is depicted as a hexagon and a furanose sugar is depicted as a pentagon. Usually an oxygen is placed at the upper right corner in pyranose and in the upper center in a furanose sugar. The thinner bonds at the top of the ring refer to the bonds as being farther away and the thicker bonds at the bottom of the ring refer to the end of the ring that is closer to the viewer.
Fischer projection
TheFischer projection
In chemistry, the Fischer projection, devised by Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates ...
is mostly used for linear monosaccharides
Monosaccharides (from Greek '' monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units ( monomers) from which all carbohydrates are built.
They are usually colorless, water ...
. At any given carbon center, vertical bond lines are equivalent to stereochemical hashed markings, directed away from the observer, while horizontal lines are equivalent to wedges, pointing toward the observer. The projection is unrealistic, as a saccharide would never adopt this multiply eclipsed conformation. Nonetheless, the Fischer projection is a simple way of depicting multiple sequential stereocenters that does not require or imply any knowledge of actual conformation. A Fischer projection will restrict a 3-D molecule to 2-D, and therefore, there are limitations to changing the configuration of the chiral centers. Fischer projections are used to determine the R and S configuration on a chiral carbon and it is done using the Cahn Ingold Prelog rules. It is a convenient way to represent and distinguish between enantiomers and diastereomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have di ...
s.
Limitations
A structural formula is a simplified model that cannot represent certain aspects of chemical structures. For example, formalized bonding may not be applicable to dynamic systems such asdelocalized bond
In theoretical chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented a ...
s. Aromaticity
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
is such a case and relies on convention to represent the bonding. Different styles of structural formulas may represent aromaticity in different ways, leading to different depictions of the same chemical compound. Another example is formal double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betwee ...
s where the electron density is spread outside the formal bond, leading to partial double bond character and slow inter-conversion at room temperature. For all dynamic effects, temperature will affect the inter-conversion rates and may change how the structure should be represented. There is no explicit temperature associated with a structural formula, although many assume that it would be standard temperature.
See also
*Molecular graph
In chemical graph theory and in mathematical chemistry, a molecular graph or chemical graph is a representation of the structural formula of a chemical compound in terms of graph theory. A chemical graph is a labeled graph whose vertices corresp ...
* Chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbol ...
* Valency interaction formula The Valency Interaction Formula, or VIF provides a way of drawing or interpreting the molecular structural formula based on molecular orbital theory. Valency Points, VP, dots drawn on a page, represent valence orbitals. Valency Interactions, VI, ...
* Side chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called the "main chain" or backbone. The side chain is a hydrocarbon branching element of a molecule that is attached to a ...
* Chemical structure
A chemical structure determination includes a chemist's specifying the molecular geometry and, when feasible and necessary, the electronic structure of the target molecule or other solid. Molecular geometry refers to the spatial arrangement of ...
Notes
References
External links
The Importance of Structural Formulas
*
{{DEFAULTSORT:Structural Formula Chemical formulas Chemical structures