|
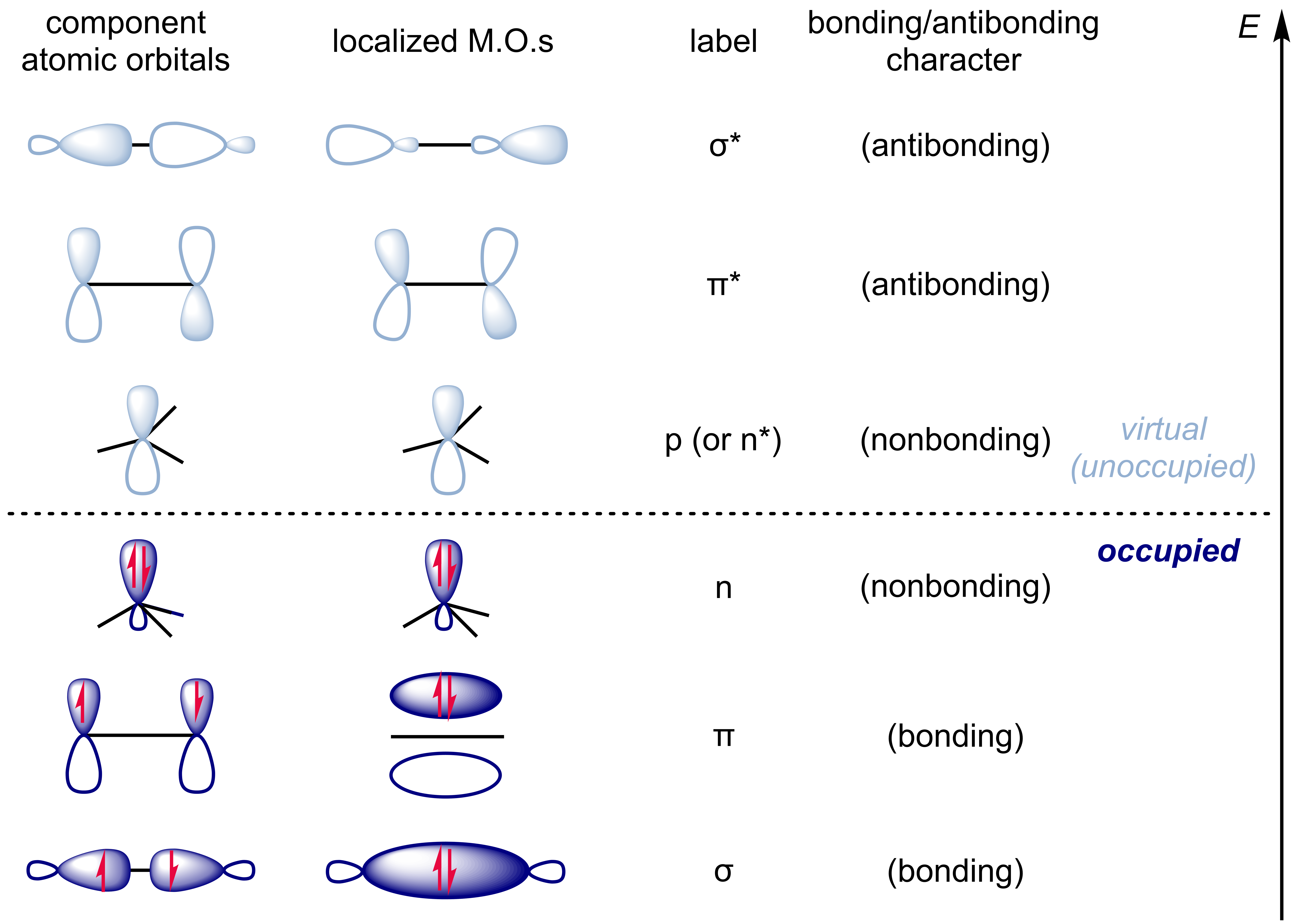
Valency Interaction Formula
The Valency Interaction Formula, or VIF provides a way of drawing or interpreting the molecular structural formula based on molecular orbital theory. Valency Points, VP, dots drawn on a page, represent valence orbitals. Valency Interactions, VI, that connect the dots, show interactions between these valence orbitals. Theory was developed by Turkish quantum chemist Oktay Sinanoğlu in the early 1980s and first published in 1983. The theory was like a new language of quantum mechanics by the exact definition of Hilbert space. It was also the solution of the problem that Paul Dirac was trying to solve at the time of his death in 1984, which concerned the hidden symmetries in Hilbert space which were responsible for the accidental degeneracies not arising from a spatial symmetry, that was about the higher symmetries of Hilbert space) Sinanoğlu showed that the solution was possible only when the topology tool was used. This VIF theory also connected both delocalized and localized mol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Formula
The structural formula of a chemical compound is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are possibly arranged in the real three-dimensional space. The chemical bonding within the molecule is also shown, either explicitly or implicitly. Unlike other chemical formula types, which have a limited number of symbols and are capable of only limited descriptive power, structural formulas provide a more complete geometric representation of the molecular structure. For example, many chemical compounds exist in different isomeric forms, which have different enantiomeric structures but the same molecular formula. There are multiple types of ways to draw these structural formulas such as: Lewis Structures, condensed formulas, skeletal formulas, Newman projections, Cyclohexane conformations, Haworth projections, and Fischer projections. Several systematic chemical naming formats, as in chemical databases, are used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Operator
In physics, an operator is a function over a space of physical states onto another space of physical states. The simplest example of the utility of operators is the study of symmetry (which makes the concept of a group useful in this context). Because of this, they are very useful tools in classical mechanics. Operators are even more important in quantum mechanics, where they form an intrinsic part of the formulation of the theory. Operators in classical mechanics In classical mechanics, the movement of a particle (or system of particles) is completely determined by the Lagrangian L(q, \dot, t) or equivalently the Hamiltonian H(q, p, t), a function of the generalized coordinates ''q'', generalized velocities \dot = \mathrm q / \mathrm t and its conjugate momenta: :p = \frac If either ''L'' or ''H'' is independent of a generalized coordinate ''q'', meaning the ''L'' and ''H'' do not change when ''q'' is changed, which in turn means the dynamics of the particle are still the same e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Structure
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the chemical bonding, bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as complex (chemistry), coordination compounds. The Lewis structure was named after Gilbert N. Lewis, who introduced it in his 1916 article ''The Atom and the Molecule.'' Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another (pairs of dots can be used instead of lines). Excess electrons that form lone pairs are represented as pairs of dots, and are placed next to the at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Formula
The structural formula of a chemical compound is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are possibly arranged in the real three-dimensional space. The chemical bonding within the molecule is also shown, either explicitly or implicitly. Unlike other chemical formula types, which have a limited number of symbols and are capable of only limited descriptive power, structural formulas provide a more complete geometric representation of the molecular structure. For example, many chemical compounds exist in different isomeric forms, which have different enantiomeric structures but the same molecular formula. There are multiple types of ways to draw these structural formulas such as: Lewis Structures, condensed formulas, skeletal formulas, Newman projections, Cyclohexane conformations, Haworth projections, and Fischer projections. Several systematic chemical naming formats, as in chemical databases, are used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Open Access Journal
Open access (OA) is a set of principles and a range of practices through which research outputs are distributed online, free of access charges or other barriers. With open access strictly defined (according to the 2001 definition), or libre open access, barriers to copying or reuse are also reduced or removed by applying an open license for copyright. The main focus of the open access movement is "peer reviewed research literature". Historically, this has centered mainly on print-based academic journals. Whereas non-open access journals cover publishing costs through access tolls such as subscriptions, site licenses or pay-per-view charges, open-access journals are characterised by funding models which do not require the reader to pay to read the journal's contents, relying instead on author fees or on public funding, subsidies and sponsorships. Open access can be applied to all forms of published research output, including peer-reviewed and non peer-reviewed academic journ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibonding Molecular Orbital
In chemical bonding theory, an antibonding orbital is a type of molecular orbital that weakens the chemical bond between two atoms and helps to raise the energy of the molecule relative to the separated atoms. Such an orbital has one or more nodes in the bonding region between the nuclei. The density of the electrons in the orbital is concentrated outside the bonding region and acts to pull one nucleus away from the other and tends to cause mutual repulsion between the two atoms. This is in contrast to a bonding molecular orbital, which has a lower energy than that of the separate atoms, and is responsible for chemical bonds. Diatomic molecules Antibonding molecular orbitals (MOs) are normally ''higher'' in energy than bonding molecular orbitals. Bonding and antibonding orbitals form when atoms combine into molecules. If two hydrogen atoms are initially far apart, they have identical atomic orbitals. However, as the spacing between the two atoms becomes smaller, the electron w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-bonding Orbital
A non-bonding orbital, also known as ''non-bonding molecular orbital'' (NBMO), is a molecular orbital whose occupation by electrons neither increases nor decreases the bond order between the involved atoms. Non-bonding orbitals are often designated by the letter n in molecular orbital diagrams and Molecular electronic transition, electron transition notations. Non-bonding orbitals are the equivalent in molecular orbital theory of the lone pairs in Lewis structures. The energy level of a non-bonding orbital is typically in between the lower energy of a valence shell bonding orbital and the higher energy of a corresponding antibonding orbital. As such, a non-bonding orbital with electrons would commonly be a HOMO (highest occupied molecular orbital). According to molecular orbital theory, molecular orbitals are often modeled by the linear combination of atomic orbitals. In a simple diatomic molecule such as hydrogen fluoride (chemical formula: HF), one atom may have many more elec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Invariant (mathematics)
In mathematics, an invariant is a property of a mathematical object (or a class of mathematical objects) which remains unchanged after operations or transformations of a certain type are applied to the objects. The particular class of objects and type of transformations are usually indicated by the context in which the term is used. For example, the area of a triangle is an invariant with respect to isometries of the Euclidean plane. The phrases "invariant under" and "invariant to" a transformation are both used. More generally, an invariant with respect to an equivalence relation is a property that is constant on each equivalence class. Invariants are used in diverse areas of mathematics such as geometry, topology, algebra and discrete mathematics. Some important classes of transformations are defined by an invariant they leave unchanged. For example, conformal maps are defined as transformations of the plane that preserve angles. The discovery of invariants is an important ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linear Transformation
In mathematics, and more specifically in linear algebra, a linear map (also called a linear mapping, linear transformation, vector space homomorphism, or in some contexts linear function) is a mapping V \to W between two vector spaces that preserves the operations of vector addition and scalar multiplication. The same names and the same definition are also used for the more general case of modules over a ring; see Module homomorphism. If a linear map is a bijection then it is called a . In the case where V = W, a linear map is called a (linear) ''endomorphism''. Sometimes the term refers to this case, but the term "linear operator" can have different meanings for different conventions: for example, it can be used to emphasize that V and W are real vector spaces (not necessarily with V = W), or it can be used to emphasize that V is a function space, which is a common convention in functional analysis. Sometimes the term ''linear function'' has the same meaning as ''linear map ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Orbital Theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century. In molecular orbital theory, electrons in a molecule are not assigned to individual chemical bonds between atoms, but are treated as moving under the influence of the atomic nuclei in the whole molecule. Quantum mechanics describes the spatial and energetic properties of electrons as molecular orbitals that surround two or more atoms in a molecule and contain valence electrons between atoms. Molecular orbital theory revolutionized the study of chemical bonding by approximating the states of bonded electrons—the molecular orbitals—as linear combinations of atomic orbitals (LCAO). These approximations are made by applying the density functional theory (DFT) or Hartree–Fock (HF) models to the Schrödinger equation. Molecular orbital theory and valence bond theory are the foundational ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Localized Molecular Orbitals
Localized molecular orbitals are molecular orbitals which are concentrated in a limited spatial region of a molecule, such as a specific bond or lone pair on a specific atom. They can be used to relate molecular orbital calculations to simple bonding theories, and also to speed up post-Hartree–Fock electronic structure calculations by taking advantage of the local nature of electron correlation. Localized orbitals in systems with periodic boundary conditions are known as Wannier functions. Standard ab initio quantum chemistry methods lead to delocalized orbitals that, in general, extend over an entire molecule and have the symmetry of the molecule. Localized orbitals may then be found as linear combinations of the delocalized orbitals, given by an appropriate unitary transformation. In the water molecule for example, ab initio calculations show bonding character primarily in two molecular orbitals, each with electron density equally distributed among the two O-H bonds. The locali ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paul Dirac
Paul Adrien Maurice Dirac (; 8 August 1902 – 20 October 1984) was an English theoretical physicist who is regarded as one of the most significant physicists of the 20th century. He was the Lucasian Professor of Mathematics at the University of Cambridge, a professor of physics at Florida State University and the University of Miami, and a 1933 Nobel Prize recipient. Dirac made fundamental contributions to the early development of both quantum mechanics and quantum electrodynamics. Among other discoveries, he formulated the Dirac equation which describes the behaviour of fermions and predicted the existence of antimatter. Dirac shared the 1933 Nobel Prize in Physics with Erwin Schrödinger "for the discovery of new productive forms of atomic theory". He also made significant contributions to the reconciliation of general relativity with quantum mechanics. Dirac was regarded by his friends and colleagues as unusual in character. In a 1926 letter to Paul Ehrenfest, Albert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |