
COVID-19 testing involves analyzing samples to assess the current or past presence of
SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) is a strain of coronavirus that causes COVID-19 (coronavirus disease 2019), the respiratory illness responsible for the ongoing COVID-19 pandemic. The virus previously had a ...
. The two main types of tests detect either the presence of the virus or
antibodies produced in response to infection. Molecular tests for viral presence through its molecular components are used to diagnose individual cases and to allow public health authorities to trace and contain outbreaks. Antibody tests (serology immunoassays) instead show whether someone once had the disease.
They are less useful for diagnosing current infections because antibodies may not develop for weeks after infection.
It is used to assess disease prevalence, which aids the estimation of the
infection fatality rate
In epidemiology, case fatality rate (CFR) – or sometimes more accurately case-fatality risk – is the proportion of people diagnosed with a certain disease, who end up dying of it. Unlike a disease's mortality rate, the CFR does not take int ...
.
Individual jurisdictions have adopted varied testing protocols, including whom to test, how often to test, analysis protocols, sample collection and the uses of test results.
This variation has likely significantly impacted reported statistics, including case and test numbers, case fatality rates and case demographics.
Because SARS-CoV-2 transmission occurs days after exposure (and before onset of symptoms), there is an urgent need for frequent surveillance and rapid availability of results.
Test analysis is often performed in
automated
Automation describes a wide range of technologies that reduce human intervention in processes, namely by predetermining decision criteria, subprocess relationships, and related actions, as well as embodying those predeterminations in machines ...
,
high-throughput,
medical laboratories
A medical laboratory or clinical laboratory is a laboratory where tests are conducted out on clinical specimens to obtain information about the health of a patient to aid in diagnosis, treatment, and prevention of disease. Clinical Medical labor ...
by
medical laboratory scientist
A medical laboratory scientist (MLS) or clinical laboratory scientist (CLS) or medical technologist (MT) performs diagnostic testing of blood and body fluids in clinical laboratories. The scope of a medical laboratory scientist's work begins wit ...
s.
Rapid self-tests and
point-of-care testing
Point-of-care testing (POCT or bedside testing) is defined as medical diagnostic testing at or near the point of care—that is, at the time and place of patient care. This contrasts with the historical pattern in which testing was wholly or most ...
are also available and can offer a faster and less expensive method to test for the virus although with a lower accuracy.
Methods

Positive viral tests indicate a current infection, while positive antibody tests indicate a prior infection.
Other techniques include a
CT scan, checking for elevated body temperature, checking for low blood oxygen level, and
detection by trained dogs.
Detection of the virus
Detection of the virus is usually done either by looking for the virus's inner
RNA, or pieces of protein on the outside of the virus. Tests that look for the viral
antigen
In immunology, an antigen (Ag) is a molecule or molecular structure or any foreign particulate matter or a pollen grain that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune respons ...
s (parts of the virus) are called ''antigen tests''.
There are multiple types of tests that look for the virus by detecting the presence of the virus's RNA. These are called ''nucleic acid'' or ''molecular'' tests, after
molecular biology
Molecular biology is the branch of biology that seeks to understand the molecular basis of biological activity in and between cells, including biomolecular synthesis, modification, mechanisms, and interactions. The study of chemical and physi ...
. , the most common form of molecular test is the reverse transcription polymerase chain reaction (RT-PCR) test.
Other methods used in molecular tests include
CRISPR,
isothermal nucleic acid amplification,
digital polymerase chain reaction,
microarray analysis, and
next-generation sequencing Massive parallel sequencing or massively parallel sequencing is any of several high-throughput approaches to DNA sequencing using the concept of massively parallel processing; it is also called next-generation sequencing (NGS) or second-generation ...
.
Reverse transcription polymerase chain reaction (RT-PCR) test
Polymerase chain reaction
The polymerase chain reaction (PCR) is a method widely used to rapidly make millions to billions of copies (complete or partial) of a specific DNA sample, allowing scientists to take a very small sample of DNA and amplify it (or a part of it) ...
(PCR) is a process that
amplifies (replicates) a small, well-defined segment of
DNA many hundreds of thousands of times, creating enough of it for analysis. Test samples are treated with certain chemicals
[ that allow DNA to be extracted. Reverse transcription converts RNA into DNA.
Reverse transcription polymerase chain reaction (RT-PCR) first uses reverse transcription to obtain DNA, followed by PCR to amplify that DNA, creating enough to be analyzed.]SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) is a strain of coronavirus that causes COVID-19 (coronavirus disease 2019), the respiratory illness responsible for the ongoing COVID-19 pandemic. The virus previously had a ...
, which contains only RNA. The RT-PCR process generally requires a few hours.Real-time PCR
A real-time polymerase chain reaction (real-time PCR, or qPCR) is a laboratory technique of molecular biology based on the polymerase chain reaction (PCR). It monitors the amplification of a targeted DNA molecule during the PCR (i.e., in real ...
(qPCR) A 90% specific test will correctly identify 90% of those who are uninfected, leaving 10% with a false positive result.
Samples can be obtained by various methods, including a
A 90% specific test will correctly identify 90% of those who are uninfected, leaving 10% with a false positive result.
Samples can be obtained by various methods, including a nasopharyngeal swab
A nasopharyngeal swab is a device used for collecting a sample of nasal secretions from the back of the nose and throat. The sample is then analyzed for the presence of organisms or other clinical markers for disease. This diagnostic method is c ...
, sputum (coughed up material),nasopharyngeal swab
A nasopharyngeal swab is a device used for collecting a sample of nasal secretions from the back of the nose and throat. The sample is then analyzed for the presence of organisms or other clinical markers for disease. This diagnostic method is c ...
for COVID-19 testing
File:Infektionsschutzzentrum im Rautenstrauch-Joest-Museum, Köln-6306 (cropped).jpg, Demonstration of a throat swab
In medicine, sampling is gathering of matter from the body to aid in the process of a medical diagnosis and/or evaluation of an indication for treatment, further medical tests or other procedures. In this sense, the sample is the gathered matter ...
for COVID-19 testing
File:Cycler offen.JPG, A PCR machine
File:Prueba COVID.webm, Video of a nasopharyngeal swab
A nasopharyngeal swab is a device used for collecting a sample of nasal secretions from the back of the nose and throat. The sample is then analyzed for the presence of organisms or other clinical markers for disease. This diagnostic method is c ...
for COVID-19 testing
Other molecular tests
Isothermal nucleic acid amplification tests also amplify the virus's genome. They are faster than PCR because they don't involve repeated heating and cooling cycles. These tests typically detect DNA using fluorescent tag
In molecular biology and biotechnology, a fluorescent tag, also known as a fluorescent label or fluorescent probe, is a molecule that is attached chemically to aid in the detection of a biomolecule such as a protein, antibody, or amino acid. Gener ...
s, which are read out with specialized machines.
CRISPR gene editing
CRISPR gene editing (pronounced "crisper") is a genetic engineering technique in molecular biology by which the genomes of living organisms may be modified. It is based on a simplified version of the bacterial CRISPR-Cas9 antiviral defense syst ...
technology was modified to perform the detection: if the CRISPR enzyme attaches to the sequence, it colors a paper strip. The researchers expect the resulting test to be cheap and easy to use in point-of-care settings. The test amplifies RNA directly, without the RNA-to-DNA conversion step of RT-PCR.
Antigen tests


 An
An antigen
In immunology, an antigen (Ag) is a molecule or molecular structure or any foreign particulate matter or a pollen grain that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune respons ...
is the part of a pathogen
In biology, a pathogen ( el, πάθος, "suffering", "passion" and , "producer of") in the oldest and broadest sense, is any organism or agent that can produce disease. A pathogen may also be referred to as an infectious agent, or simply a germ ...
that elicits an immune response
An immune response is a reaction which occurs within an organism for the purpose of defending against foreign invaders. These invaders include a wide variety of different microorganisms including viruses, bacteria, parasites, and fungi which could ...
. Antigen tests look for antigen protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s from the viral surface. In the case of a coronavirus, these are usually proteins from the surface spikes.COVID-19 rapid antigen test
COVID-19 rapid antigen tests or RATs, also frequently called COVID-19 lateral flow tests or LFTs, are rapid antigen tests used to detect SARS-CoV-2 infection (COVID-19). They are quick to implement with minimal training, cost a fraction of other ...
s are lateral flow immunoassay
An immunoassay (IA) is a biochemical test that measures the presence or concentration of a macromolecule or a small molecule in a solution through the use of an antibody (usually) or an antigen (sometimes). The molecule detected by the immunoass ...
s that detect the presence of a specific viral antigen
In immunology, an antigen (Ag) is a molecule or molecular structure or any foreign particulate matter or a pollen grain that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune respons ...
, which indicates current viral infection. Antigen tests produce results quickly (within approximately 15–30 minutes), and most can be used at the point-of-care or as self-tests. Self-tests are rapid tests that can be taken at home or anywhere, are easy to use, and produce rapid results. Antigen tests can be performed on nasopharyngeal, nasal swab, or saliva specimens.RT-PCR
Reverse transcription polymerase chain reaction (RT-PCR) is a laboratory technique combining reverse transcription of RNA into DNA (in this context called complementary DNA or cDNA) and amplification of specific DNA targets using polymerase ch ...
tests are accurate but require too much time, energy and trained personnel to run the tests.Deborah Birx
Deborah Leah Birx (born April 4, 1956) is an American physician and diplomat who served as the White House Coronavirus Response Coordinator under President Donald Trump from 2020 to 2021. Birx specializes in HIV/AIDS immunology, vaccine resear ...
, head of the White House Coronavirus Task Force
The White House Coronavirus Task Force was the United States Department of State task force during the Trump administration that "coordinate and overs wthe administration's efforts to monitor, prevent, contain, and mitigate the spread" of cor ...
, said on 17 April 2020. "But there might be with the antigen test."
Samples may be collected via nasopharyngeal swab, a swab of the anterior nares, or from saliva (obtained by various methods including ''lollipop tests'' for children).
Antibody tests
The body responds to a viral infection by producing antibodies that help neutralize the virus.mortality rate
Mortality rate, or death rate, is a measure of the number of deaths (in general, or due to a specific cause) in a particular population, scaled to the size of that population, per unit of time. Mortality rate is typically expressed in units of d ...
.[
The most notable antibodies are ]IgM
Immunoglobulin M (IgM) is one of several isotypes of antibody (also known as immunoglobulin) that are produced by vertebrates. IgM is the largest antibody, and it is the first antibody to appear in the response to initial exposure to an antig ...
and IgG
Immunoglobulin G (Ig G) is a type of antibody. Representing approximately 75% of serum antibodies in humans, IgG is the most common type of antibody found in blood circulation. IgG molecules are created and released by plasma B cells. Each IgG ...
. IgM antibodies are generally detectable several days after initial infection, although levels over the course of infection and beyond are not well characterized.
Antibody Test Types
= Rapid diagnostic test (RDT)
=
RDTs typically use a small, portable, positive/negative lateral flow assay that can be executed at point of care. RDTs may process blood samples, saliva samples, or nasal swab fluids. RDTs produce colored lines to indicate positive or negative results.
= Enzyme-linked immunosorbent assay (ELISA)
=
ELISA
The enzyme-linked immunosorbent assay (ELISA) (, ) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay uses a solid-phase type of enzyme immunoassay (EIA) to detect the presen ...
s can be qualitative or quantitative and generally require a lab. These tests usually use whole blood
Whole blood (WB) is human blood from a standard blood donation. It is used in the treatment of massive bleeding, in exchange transfusion, and when people donate blood to themselves. One unit of whole blood (~517 mls) brings up hemoglobin level ...
, plasma, or serum samples. A plate is coated with a viral protein, such as a SARS-CoV-2 spike protein. Samples are incubated with the protein, allowing any antibodies to bind to it. The antibody-protein complex can then be detected with another wash of antibodies that produce a color/fluorescent readout.[
]
= Neutralization assay
=
Neutralization assays assess whether sample antibodies prevent viral infection in test cells.[
]
= Chemiluminescent immunoassay
=
Chemiluminescent immunoassay
An immunoassay (IA) is a biochemical test that measures the presence or concentration of a macromolecule or a small molecule in a solution through the use of an antibody (usually) or an antigen (sometimes). The molecule detected by the immunoass ...
s are quantitative lab tests. They sample blood, plasma, or serum. Samples are mixed with a known viral protein, buffer reagents and specific, enzyme-labeled antibodies. The result is luminescent. A chemiluminescent microparticle immunoassay uses magnetic, protein-coated microparticles. Antibodies react to the viral protein, forming a complex. Secondary enzyme-labeled antibodies are added and bind to these complexes. The resulting chemical reaction produces light. The radiance is used to calculate the number of antibodies. This test can identify multiple types of antibodies, including IgG, IgM, and IgA.[
]
Neutralizing vis-à-vis binding antibodies
Most if not all large scale COVID-19 antibody testing looks for binding antibodies only and does not measure the more important neutralizing antibodies
A neutralizing antibody (NAb) is an antibody that defends a cell from a pathogen or infectious particle by neutralizing any effect it has biologically. Neutralization renders the particle no longer infectious or pathogenic.
Neutralizing antibod ...
(NAb).[
It is expected that binding antibodies imply the presence of NAbs][ and for many viral diseases total antibody responses correlate somewhat with NAb responses][ An additional source of uncertainty is that even if NAbs are present, viruses such as HIV can evade NAb responses.][
Studies have indicated that NAbs to the original ]SARS
Severe acute respiratory syndrome (SARS) is a viral respiratory disease of zoonotic origin caused by the severe acute respiratory syndrome coronavirus (SARS-CoV or SARS-CoV-1), the first identified strain of the SARS coronavirus species, ''seve ...
virus (the predecessor to the current SARS-CoV-2) can remain active for two years and are gone after six years.Memory B cell
In immunology, a memory B cell (MBC) is a type of B lymphocyte that forms part of the adaptive immune system. These cells develop within germinal centers of the secondary lymphoid organs. Memory B cells circulate in the blood stream in a quiesc ...
s and Memory T cell
Memory T cells are a subset of T lymphocytes that might have some of the same functions as memory B cells. Their lineage is unclear.
Function
Antigen-specific memory T cells specific to viruses or other microbial molecules can be found in both ...
s can last much longer and may have the ability to reduce reinfection severity.[
File:MINISTRO DE DEFENSA SUPERVISÓ TOMA DE PRUEBAS DE COVID-19 A COMERCIANTES DEL MERCADO CAQUETÁ (49833483173).jpg, A point of care test in Peru. A blood droplet is collected by a pipette.
File:MINISTRO DE DEFENSA SUPERVISÓ TOMA DE PRUEBAS DE COVID-19 A COMERCIANTES DEL MERCADO CAQUETÁ (49834325922).jpg, Blood from pipette is then placed onto a COVID-19 ]rapid diagnostic test
A rapid diagnostic test (RDT) is a medical diagnostic test that is quick and easy to perform. RDTs are suitable for preliminary or emergency medical screening and for use in medical facilities with limited resources. They also allow point-of-care ...
device.
File:IgGIgM-Covid19-Test.jpg, The rapid diagnostic test shows reactions of IgG
Immunoglobulin G (Ig G) is a type of antibody. Representing approximately 75% of serum antibodies in humans, IgG is the most common type of antibody found in blood circulation. IgG molecules are created and released by plasma B cells. Each IgG ...
and IgM
Immunoglobulin M (IgM) is one of several isotypes of antibody (also known as immunoglobulin) that are produced by vertebrates. IgM is the largest antibody, and it is the first antibody to appear in the response to initial exposure to an antig ...
antibodies.
Other tests
Sniff tests
Sudden loss of smell can be used to screen people on a daily basis for COVID-19. A study by the National Institutes of Health showed that those infected with SARS-CoV-2 could not smell a 25% mixture of ethanol and water. Because various conditions can lead to the loss of the sense of smell, a sniff test would not be definitive but indicate the need for a PCR test. Because the loss of the sense of smell shows up before other symptoms, there has been a call for widespread sniff testing. Health care bureaucracies have generally ignored sniff tests even though they are quick, easy and capable of being self-administered daily. This has led some medical journals to write editorials supporting the adoption of sniff testing.
Imaging
Typical visible features on CT initially include bilateral multilobar ground-glass opacities
Ground-glass opacity (GGO) is a finding seen on chest x-ray (radiograph) or computed tomography (CT) imaging of the lungs. It is typically defined as an area of hazy opacification (x-ray) or increased attenuation (CT) due to air displacement ...
with a peripheral or posterior distribution.crazy paving
Crazy paving is a means of hard-surfacing used outdoors, most frequently in gardens. Paving stones of irregular size and shape are laid in a haphazard manner sometimes with mortar filling the gaps between.
The method originated in ancient R ...
, and consolidation may develop as the disease evolves.chest x-rays
A chest radiograph, called a chest X-ray (CXR), or chest film, is a Projectional radiography, projection radiograph of the chest used to diagnose conditions affecting the chest, its contents, and nearby structures. Chest radiographs are the most ...
are not recommended for diagnosing COVID-19. Radiologic findings in COVID-19 lack specificity.
Article body
Serology (CoLab score) tests
The standard blood test (quick scan) taken at the emergency room measures different values. By use of the blood quick scan the CoLab score is calculated with a developed algorithm based on how the coronavirus causes changes in the blood. The software is intended for use in emergency rooms to quickly rule out the presence of the disease in incoming patients. A not negative result is followed by a PCR (polymerase chain reaction
The polymerase chain reaction (PCR) is a method widely used to rapidly make millions to billions of copies (complete or partial) of a specific DNA sample, allowing scientists to take a very small sample of DNA and amplify it (or a part of it) ...
) or LAMP (loop-mediated isothermal amplification
Loop-mediated isothermal amplification (LAMP) is a single-tube technique for the amplification of DNA and a low-cost alternative to detect certain diseases. Reverse transcription loop-mediated isothermal amplification (RT-LAMP) combines LAMP with ...
) test.
Breath tests
The breath test by a Coronavirus breathalyzer
A coronavirus breathalyzer is a diagnostic medical device enabling the user to test with 90% or greater accuracy the presence of severe acute respiratory syndrome coronavirus 2 in an exhaled breath.Anthes, E."A Covid Test as Easy as Breathing" Th ...
is a pre-screening test for people who have no or mild symptoms of COVID-19. A not negative result is followed by a PCR or LAMP test.
Animals
In May 2021, Reuters reported that Dutch researchers at Wageningen University
Wageningen University & Research (also known as Wageningen UR; abbreviation: WUR) is a public university in Wageningen, Netherlands, specializing in life sciences with a focus on agriculture, technical and engineering subjects. It is a globally ...
had shown that trained bees could detect the virus in infected samples in seconds and this could benefit countries where test facilities are in short supply. A two-month study by the Necker-Cochin hospital Paris in conjunction with the French national veterinary school reported in May 2021 that dogs were more reliable than current lateral flow tests.
Researchers in Paris in March 2022 reported in a preprint not yet peer-reviewed that trained dogs were very effective for rapidly detecting the presence of SARS-Cov2 in people, whether displaying symptoms or not. The dogs were presented with sweat samples to smell from 335 people, of whom 78 with symptoms and 31 without tested positive by PCR. The dogs detected 97% of the symptomatic and 100% of the asymptomatic infections. They were 91% accurate at identifying volunteers who were not infected, and 94% accurate at ruling out the infection in people without symptoms. The authors said "Canine testing is non-invasive and provides immediate and reliable results.Further studies will be focused on direct sniffing by dogs to evaluate sniffer dogs for mass pre-test in airports, harbors, railways stations, cultural activities or sporting events."
Functional assays
Tollotest is a molecular test that detects the activity of a SARS-CoV2 protease, which is a biomarker for active infection.
History
 In January 2020, scientists from China published the first
In January 2020, scientists from China published the first genetic sequence
A nucleic acid sequence is a succession of bases signified by a series of a set of five different letters that indicate the order of nucleotides forming alleles within a DNA (using GACT) or RNA (GACU) molecule. By convention, sequences are us ...
s of SARS-CoV-2 via the GISAID
GISAID (Global Initiative on Sharing Avian Influenza Data) is a global science initiative and primary source established in 2008 that provides open access to genomic data of influenza viruses and the coronavirus responsible for the COVID-19 pan ...
initiative, a program that had handled mostly genetic sequence data from animal-borne influenzas.Shortages
In economics, a shortage or excess demand is a situation in which the demand for a product or service exceeds its supply in a market. It is the opposite of an excess supply (surplus).
Definitions
In a perfect market (one that matches ...
of reagent and other testing supplies became a bottleneck
Bottleneck literally refers to the narrowed portion (neck) of a bottle near its opening, which limit the rate of outflow, and may describe any object of a similar shape. The literal neck of a bottle was originally used to play what is now known as ...
for mass testing in the EU, the UK and the US.
Testing protocols
Drive-through testing
In drive-through testing, the person undergoing testing remains in a vehicle while a healthcare professional approaches the vehicle and obtains a sample, all while taking appropriate precautions such as wearing personal protective equipment
Personal protective equipment (PPE) is protective clothing, helmets, goggles, or other garments or equipment designed to protect the wearer's body from injury or infection. The hazards addressed by protective equipment include physical, e ...
(PPE). Drive-through centers helped South Korea accelerate its testing program.
Home collection
 In Hong Kong test subjects can stay home and receive a specimen tube. They spit into it, return it and later get the result.
In Hong Kong test subjects can stay home and receive a specimen tube. They spit into it, return it and later get the result.
Pooled testing
Pooled testing can improve turnaround time, by combining a number of samples to be tested together. If the pool result is negative, all samples are negative. If the test result is positive, samples will need to be individually tested.Rambam Hospital
Rambam Health Care Campus ( he, רמב"ם - הקריה הרפואית לבריאות האדם) commonly called Rambam Hospital, is a teaching hospital in the Bat Galim neighborhood of Haifa, Israel founded in 1938, 10 years before the establishme ...
developed a method for testing samples from 64 patients simultaneously, by pooling the samples and only testing further if the combined sample was positive. Pool testing was then adopted in Israel, Germany, Ghana South Korea, Nebraska
Nebraska () is a state in the Midwestern region of the United States. It is bordered by South Dakota to the north; Iowa to the east and Missouri to the southeast, both across the Missouri River; Kansas to the south; Colorado to the sout ...
, China and the Indian states of Uttar Pradesh
Uttar Pradesh (; , 'Northern Province') is a state in northern India. With over 200 million inhabitants, it is the most populated state in India as well as the most populous country subdivision in the world. It was established in 1950 ...
, West Bengal
West Bengal (, Bengali: ''Poshchim Bongo'', , abbr. WB) is a state in the eastern portion of India. It is situated along the Bay of Bengal, along with a population of over 91 million inhabitants within an area of . West Bengal is the fou ...
, Punjab
Punjab (; Punjabi: پنجاب ; ਪੰਜਾਬ ; ; also romanised as ''Panjāb'' or ''Panj-Āb'') is a geopolitical, cultural, and historical region in South Asia, specifically in the northern part of the Indian subcontinent, comprising a ...
, Chhattisgarh and Maharashtra.
Open source, multiplexed designs released by Origami Assays can test as many as 1122 patient samples using only 93 assays. These balanced designs can be run in small laboratories without robotic liquid handlers.
Multi-tiered testing
One study proposed a rapid immune response assay as a screening test, with a confirmatory nucleic acid test for diagnosis, followed by a rapid antibody test to determine course of action and assess population exposure/herd immunity.
Required volume
Required testing levels are a function of disease spread. The more the cases, the more tests are needed to manage the outbreak. COVID-19 tends to grow exponentially at the beginning of an outbreak, meaning that the number of required tests initially also grows exponentially. If properly targeted testing grows more rapidly than cases, it can be contained.
WHO recommends increasing testing until fewer than 10% are positive in any given jurisdiction.
United States
 Economist Paul Romer reported that the US has the technical capacity to scale up to 20 million tests per day, which is his estimate of the scale needed to fully remobilize the economy.
Economist Paul Romer reported that the US has the technical capacity to scale up to 20 million tests per day, which is his estimate of the scale needed to fully remobilize the economy.Pacific Biosciences
Pacific Biosciences of California, Inc. (aka PacBio) is an American biotechnology company founded in 2004 that develops and manufactures systems for gene sequencing and some novel real time biological observation. PacBio describes its platfor ...
ThermoFisher Scientific
Thermo Fisher Scientific Inc. is an American supplier of scientific instrumentation, reagents and consumables, and software services. Based in Waltham, Massachusetts, Thermo Fisher was formed through the merger of Thermo Electron and Fisher ...
.false negative
A false positive is an error in binary classification in which a test result incorrectly indicates the presence of a condition (such as a disease when the disease is not present), while a false negative is the opposite error, where the test resul ...
rate and a 1% false positive
A false positive is an error in binary classification in which a test result incorrectly indicates the presence of a condition (such as a disease when the disease is not present), while a false negative is the opposite error, where the test resul ...
rate. The average person would be tested roughly every two weeks. Those who tested positive would go into quarantine. Romer's simulation indicated that the fraction of the population that is infected at any given time (known as the attack rate In epidemiology, the attack rate is the proportion of an at-risk population that contracts the disease during a specified time interval. It is used in hypothetical predictions and during actual outbreaks of disease. An at-risk population is defined ...
) peaks reaches roughly 8% in about thirty days before gradually declining, in most runs reaching zero at 500 days, with cumulative prevalence remaining below 20%.
Snapshot mass-testing
A study found that, despite possibly suboptimal implementation, the snapshot mass-testing approach conducted by Slovakia
Slovakia (; sk, Slovensko ), officially the Slovak Republic ( sk, Slovenská republika, links=no ), is a landlocked country in Central Europe. It is bordered by Poland to the north, Ukraine to the east, Hungary to the south, Austria to the s ...
by which ~80% of its population was tested for COVID-19 within a weekend at the end of October 2020 was highly efficacious, decreasing observed prevalence by 58% within one week and by 70% compared to a hypothetical scenario of no snapshot mass-testing. The significant reduction resulted from a set of complementary lockdown and quarantine measures whereby citizens who tested positive were quarantined synchronously the weeks afterwards.
Surveillance and screening of populations
As of August 2020, the WHO recognizes wastewater surveillance of SARS-CoV-2 as a potentially useful source of information on the prevalence and temporal trends of COVID-19 in communities, while highlighting that gaps in research such as viral shedding characteristics should be addressed.wastewater-based epidemiology
Wastewater-based epidemiology (or wastewater-based surveillance or sewage chemical-information mining) analyzes wastewater to determine the consumption of, or exposure to, chemicals or pathogens in a population. This is achieved by measuring chemic ...
has the potential for an early warning system and monitoring for COVID-19 infections. This may prove particularly useful once large shares of regional populations are vaccinated or recovered and don't need to conduct rapid tests while in some cases being infectious nevertheless.
Available tests
 Countries around the world developed tests independently and in partnership with others.
Countries around the world developed tests independently and in partnership with others.
Nucleic acid tests
Tests are available that look for viral DNA using either polymerase chain reaction
The polymerase chain reaction (PCR) is a method widely used to rapidly make millions to billions of copies (complete or partial) of a specific DNA sample, allowing scientists to take a very small sample of DNA and amplify it (or a part of it) ...
(PCR) or loop-mediated isothermal amplification
Loop-mediated isothermal amplification (LAMP) is a single-tube technique for the amplification of DNA and a low-cost alternative to detect certain diseases. Reverse transcription loop-mediated isothermal amplification (RT-LAMP) combines LAMP with ...
(LAMP) technology.
Tests developed in China, France, Germany, Hong Kong, Japan, the United Kingdom, and the US targeted different parts of the viral genome. WHO adopted the German system for manufacturing kits sent to low-income countries without the resources to develop their own.
PowerChek Coronavirus looks for the "E" gene shared by all beta coronaviruses, and the RdRp gene specific to SARS-CoV-2.
Abbott Laboratories' ID Now nucleic acid test uses isothermal amplification technology.molecular beacon
Molecular beacons, or molecular beacon probes, are oligonucleotide hybridization probes that can report the presence of specific nucleic acids in homogenous solutions. Molecular beacons are hairpin-shaped molecules with an internally quenched fluo ...
s".[ID NOW COVID-19](_blank)
, Instruction for Use, FDA The test kit uses the company's "toaster-size" ID Now device, which is widely deployed in the US. The device can be used in laboratories or in point of care settings, and provides results in 13 minutes or less.Primerdesign
Primerdesign is a UK-based biotechnology company that designs and sells products for quantitative real-time polymerase chain reaction (qPCR).
History
Primerdesign was founded in 2005 by Dr Jim Wicks, Dr Rob Powell and Professor Tom Brown with ...
offers its Genesig Real-Time PCR test system. Roche Molecular Systems offers the Cobas 6800/8800 systems; they are offered among others by the United Nations.
Antigen tests
 Antigen tests are readily available worldwide and have been approved by several health regulators.
Quidel's "Sofia2 SARS Antigen FIA"
Antigen tests are readily available worldwide and have been approved by several health regulators.
Quidel's "Sofia2 SARS Antigen FIA"lateral flow test
A lateral flow test (LFT), is an assay also known as a lateral flow device (LFD), lateral flow immunochromatographic assay, or rapid test. It is a simple device intended to detect the presence of a target substance in a liquid sample without the ...
that uses monoclonal antibodies to detect the virus's nucleocapsid (N) protein.[Sofia 2 SARS Antigen FIA](_blank)
Instructions for Use, FDA.gov The result is read out by the company's Sofia2 device using immunofluorescence.false positive
A false positive is an error in binary classification in which a test result incorrectly indicates the presence of a condition (such as a disease when the disease is not present), while a false negative is the opposite error, where the test resul ...
s and false negative
A false positive is an error in binary classification in which a test result incorrectly indicates the presence of a condition (such as a disease when the disease is not present), while a false negative is the opposite error, where the test resul ...
s found in clinical trials were higher than the rate claimed by the packaging. Over 1 billion tests from the company have been distributed in the UK, with £3 billion in funding as part of Operation Moonshot, and the MHRK has authorized exceptional use until at least 28 August 2021.Liverpool
Liverpool is a city and metropolitan borough in Merseyside, England. With a population of in 2019, it is the 10th largest English district by population and its metropolitan area is the fifth largest in the United Kingdom, with a populat ...
.
Serology (antibody) tests
Antibodies are usually detectable 14 days after the onset of the infection. Multiple jurisdictions survey their populations using these tests. The test requires a blood sample.
Private US labs including Quest Diagnostics
Quest Diagnostics is an American clinical laboratory. A Fortune 500 company, Quest operates in the United States, Puerto Rico, Mexico, and Brazil. Quest also maintains collaborative agreements with various hospitals and clinics across the globe ...
and LabCorp offer antibody testing upon request.
Certain antibody tests are available in several European countries and also in the US.
Roche offers a selective ELISA
The enzyme-linked immunosorbent assay (ELISA) (, ) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay uses a solid-phase type of enzyme immunoassay (EIA) to detect the presen ...
serology test.
A summary review in BMJ has noted that while some "serological tests … might be cheaper and easier to implement at the point of care han RT-PCR, and such testing can identify previously infected individuals, "caution is warranted … using serological tests for … epidemiological surveillance". The review called for higher quality studies assessing accuracy with reference to a standard of "RT-PCR performed on at least two consecutive specimens, and, when feasible, includ ngviral cultures."
Accuracy
Accuracy is measured in terms of specificity and selectivity. Test errors can be false positives (the test is positive, but the virus is not present) or false negatives, (the test is negative, but the virus is present).
Sensitivity and specificity
Sensitivity indicates whether the test accurately identifies whether the virus is present. Each test requires a minimum level of viral load
Viral load, also known as viral burden, is a numerical expression of the quantity of virus in a given volume of fluid, including biological and environmental specimens. It is not to be confused with viral titre or viral titer, which depends on the ...
in order to produce a positive result. A 90% sensitive test will correctly identify 90% of infections, missing the other 10% (a false negative). Even relatively high sensitivity rates can produce high rates of false negatives in populations with low incidence rates. A 90% specific test will correctly identify 90% of those who are uninfected, leaving 10% with a false positive result.
Low-specificity tests have a low positive predictive value (PPV) when prevalence is low. For example, suppose incidence is 5%. Testing 100 people at random using a test that has a specificity of 95% would yield on average 5 people who are actually negative who would incorrectly test positive. Since 5% of the subjects actually are positive, another five would also test positive correctly, totaling 10 positive results. Thus, the PPV is 50%, an outcome no different from a coin toss. In this situation, assuming that the result of a second test is
A 90% specific test will correctly identify 90% of those who are uninfected, leaving 10% with a false positive result.
Low-specificity tests have a low positive predictive value (PPV) when prevalence is low. For example, suppose incidence is 5%. Testing 100 people at random using a test that has a specificity of 95% would yield on average 5 people who are actually negative who would incorrectly test positive. Since 5% of the subjects actually are positive, another five would also test positive correctly, totaling 10 positive results. Thus, the PPV is 50%, an outcome no different from a coin toss. In this situation, assuming that the result of a second test is independent
Independent or Independents may refer to:
Arts, entertainment, and media Artist groups
* Independents (artist group), a group of modernist painters based in the New Hope, Pennsylvania, area of the United States during the early 1930s
* Independ ...
of the first test, retesting those with a first positive result increases the PPV to 94.5%, meaning that only 4.5% of the second tests would return the incorrect result, on average less than 1 incorrect result.
Causes of test error
The time course of infection affects the accuracy of some tests. Samples may be collected before the virus has had a chance to establish itself or after the body has begun to eliminate it. A May 2020 review of PCR-RT testing found that the median probability of a false-negative result decreased from 100% on day 1, to 67% on day 4. On the day of symptom onset, the probability was 38%, which decreased to 20% 3 days later.
PCR-based test
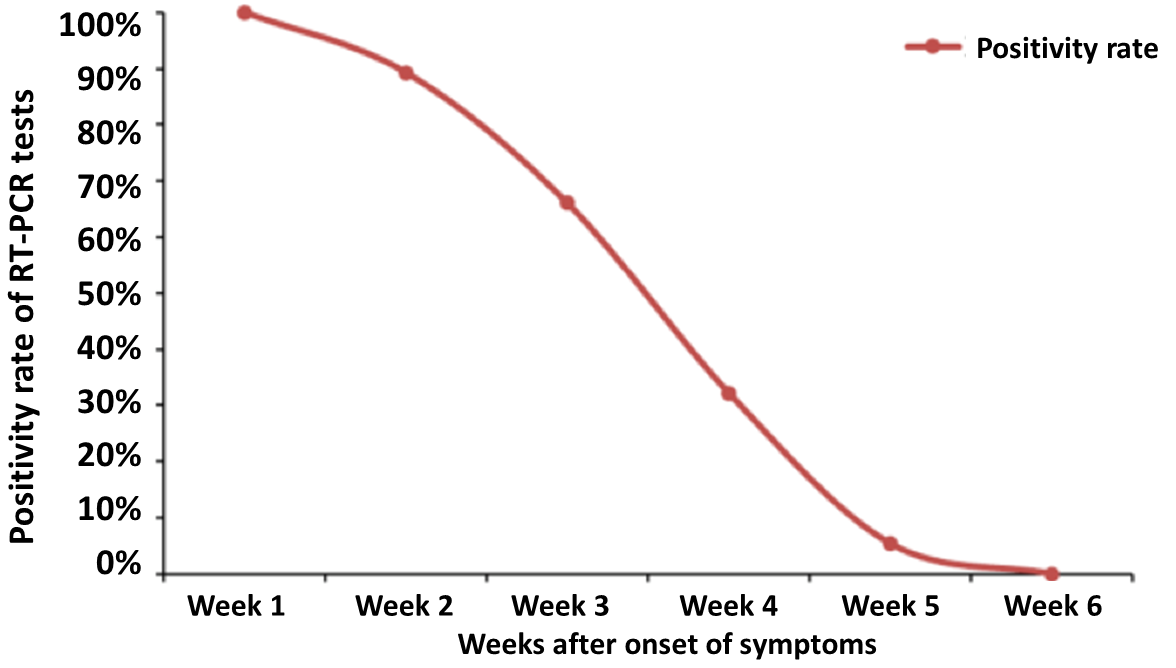 RT-PCR is the most commonly-used diagnostic test. PCR tests by nasopharyngeal swab have a sensitivity of 73%, but systematic analysis of specificity has not been determined due to the lack of PCR studies with a control group.
In one study sensitivity was highest at week one (100%), followed by 89.3%, 66.1%, 32.1%, 5.4% and zero by week six since symptom onset.
RT-PCR is the most commonly-used diagnostic test. PCR tests by nasopharyngeal swab have a sensitivity of 73%, but systematic analysis of specificity has not been determined due to the lack of PCR studies with a control group.
In one study sensitivity was highest at week one (100%), followed by 89.3%, 66.1%, 32.1%, 5.4% and zero by week six since symptom onset.Dr. Anthony Fauci
Anthony Stephen Fauci (; born December 24, 1940) is an American physician-scientist and immunologist serving as the director of the National Institute of Allergy and Infectious Diseases (NIAID) and the chief medical advisor to the president ...
of the US CDC indicated that positive results obtained from RT-PCR tests run at more than 35 cycles were almost always "just dead nucleotides". On August 29, 2020, the New York Times reported that, "In three sets of testing data that include cycle thresholds, compiled by officials in Massachusetts, New York and Nevada … most tests set the limit at 40 ycles a few at 37" and that the CDC was examining the use of cycle threshold measures "for policy decisions," On July 21, 2021, the CDC, in their "Real-Time RT-PCR Diagnostic Pan: Instructions for Use", indicated tests results should be determined at 40 cycles.
A Dutch CDC-led laboratory investigation compared 7 PCR kits.University of Oxford
, mottoeng = The Lord is my light
, established =
, endowment = £6.1 billion (including colleges) (2019)
, budget = £2.145 billion (2019–20)
, chancellor ...
's Centre for Evidence-Based Medicine
The Centre for Evidence-Based Medicine (CEBM), based in the Nuffield Department of Primary Care Health Sciences at the University of Oxford, is an academic-led centre dedicated to the practice, teaching, and dissemination of high quality evidenc ...
(CEBM) has pointed to mounting evidence that "a good proportion of 'new' mild cases and people re-testing positives via RT-PCR after quarantine or discharge from hospital are not infectious, but are simply clearing harmless virus particles which their immune system has efficiently dealt with", and have called for "an international effort to standardize and periodically calibrate testing".
Isothermal nucleic amplification test
One study reported that the ID Now COVID-19 test showed sensitivity of 85.2%. Abbott responded that the issue could have been caused by analysis delays.
Confirmatory testing
The WHO recommends countries that do not have testing capacity and national laboratories with limited experience on COVID-19 send their first five positives and the first ten negative COVID-19 samples to one of the 16 WHO reference laboratories for confirmatory testing. Out of the sixteen reference laboratories, seven are in Asia, five in Europe, two in Africa, one in North America and one in Australia.
National or regional responses
Iceland
Iceland managed the pandemic with aggressive contact tracing, inbound travel restrictions, testing, and quarantining, but with less aggressive lock-downs.
India
Italy
Researchers tested the entire population of Vo'
Vo' (or Vo' Euganeo; sometimes incorrectly spelled Vò or Vò Euganeo) is a ''comune'' (municipality) in the Province of Padua in the Italian Veneto region, located about west of Venice and about southwest of Padua, in the western end of the Eug ...
, the site of Italy's first COVID-19 death. They tested about 3,400 people twice, at an interval of ten days. About half the people testing positive had no symptoms. All discovered cases were quarantined. Along with restricting travel to the commune, new infections were eliminated.
Japan
Unlike other Asian countries, Japan did not experience a pandemic of SARS
Severe acute respiratory syndrome (SARS) is a viral respiratory disease of zoonotic origin caused by the severe acute respiratory syndrome coronavirus (SARS-CoV or SARS-CoV-1), the first identified strain of the SARS coronavirus species, ''seve ...
or MERS
Middle East respiratory syndrome (MERS) is a viral respiratory infection caused by ''Middle East respiratory syndrome–related coronavirus'' (MERS-CoV). Symptoms may range from none, to mild, to severe. Typical symptoms include fever, cough, ...
, so the country's PCR testing system was not well developed.Wuhan
Wuhan (, ; ; ) is the capital of Hubei Province in the People's Republic of China. It is the largest city in Hubei and the most populous city in Central China, with a population of over eleven million, the ninth-most populous Chinese city an ...
and identified conditions leading to clusters (closed spaces, crowded spaces and close-contact), and asked people to avoid them.[
As of 18 July, Japan's daily PCR testing capacity was about 32,000, more than three times the 10,000 cases as of April. When the antigen test is added to it, the number is about 58,000. The number of tests per 1,000 people in the United States is about 27 times that of Japan, the UK is 20 times, Italy is 8 times, and South Korea is twice (as of 26 July).
The number of those infected with coronavirus and inpatients has increased in July, but the number of serious cases has not increased. This is thought to be due to the proper testing of those infected in July compared to those in April. In April, the number of tests could not catch up with the increase in the number of infected people, and the test standards were strict, so the test positive rate exceeded 30% at the peak. It means that there were quite a few cases where the those infected was not PCR tested. It is thought that the severe case was preferentially tested though there were a lot of mild cases and asymptomatic carriers mainly in the young during the first wave. In other words, it became possible to grasp the actual situation of infection much better than before by strengthening the testing system. At the end of July, accommodation facilities for mild and asymptomatic carriers became full, and the authorities requested hospitals to prepare beds for the mild. However, it became difficult to treat patients with other illnesses and to maintain the ICU system including the staff due to the occupation of hospital beds by patients with mild symptoms.
]
Russia
On 27 April 2020, Russia tested 3 million people and had 183,000 positive results.Anna Popova
Anna Yuryevna Popova (russian: Анна Юрьевна Попова; born October 18, 1960, Rostov-on-Don, RSFSR, USSR) is a Russian physician and public health official. She is serving as head of the Federal Service for Supervision of Consumer ...
, head of Federal Service for Surveillance in Healthcare (Roszdravnadzor) stated that 506 laboratories were testing; that 45% of those who tested positive had no symptoms; that 5% of patients had a severe form; and 40% of infections were from family members. Illness improved from six days to one day after symptoms appeared. Antibody testing was carried out on 3,200 Moscow doctors, finding 20% immunity.
Singapore
With contact tracing, inbound travel restrictions, testing, and quarantining, Singapore
Singapore (), officially the Republic of Singapore, is a sovereign island country and city-state in maritime Southeast Asia. It lies about one degree of latitude () north of the equator, off the southern tip of the Malay Peninsula, bor ...
arrested the initial spread without complete lockdown.
Slovakia
In late October 2020 Slovakia tested 3.62 million people in a weekend, from a population of 5.4m, representing 67% of the total (or 82% of the adult population), 38,359 tested positive, representing 1.06% of those tested. The government considered the mass test would significantly assist in controlling the virus and avoid a lockdown and may repeat the exercise at a later date.
South Korea
South Korea's broad testing approach helped reduce spread. Testing capacity, largely in private sector labs, was built up over several years by the South Korean government in the early 2000s.
The government exploited the resident registration number
In the Republic of Korea, a resident registration number (RRN) (; romanized: ) is a 13-digit number issued to all residents of South Korea regardless of nationality. Similar to national identification numbers in other countries, it is used to id ...
(RRN) system. Authorities mobilized young men who were eligible for military service as social service agents, security and public health doctors. Public health doctors were mainly dispatched to public health centers and life treatment centers where mildly ill patients were accommodated. They performed PCR tests and managed mild patients. Social service agents worked in pharmacies to fill staff shortages. Korea's 10k PCR tests per million residents was the world's highest as of 13 April rising to 20k by mid-June. Twenty-seven Korean companies exported test kits worth $48.6 million in March, and were asked to provide test kits or humanitarian assistance by more than 120 countries. Korean authorities set up a treatment center to isolate and manage patients with asymptomatic and minor illnesses in one facility in order to vacate hospital beds for the more severely ill.
Centers were sited mainly at national facilities and corporate training centers. The failure of Korea's MERS quarantine in May 2015 left Korea more prepared for COVID-19 than countries that did not face that pandemic. Then President Park Geun-hye
Park Geun-hye (; ; often in English ; born 2 February 1952) is a South Korean politician who served as the 11th president of South Korea from 2013 to 2017, until she was impeached and convicted on related corruption charges.
Park was the fi ...
allowed Korean CDC-approved private sector testing for infectious diseases in 2016. Korea already had a system for isolating, testing and treating infectious disease patients separately from others. Patients with respiratory illness but no epidemiological relevance were treated at the National Hospital, and those with epidemiological relevance were treated at selected clinics.
Taiwan
Health insurance IDs and national identification card
An identity document (also called ID or colloquially as papers) is any document that may be used to prove a person's identity. If issued in a small, standard credit card size form, it is usually called an identity card (IC, ID card, citizen ca ...
numbers were used to trace contacts.
United Arab Emirates
In January 2021, the COVID-19 testing results of the UAE
The United Arab Emirates (UAE; ar, اَلْإِمَارَات الْعَرَبِيَة الْمُتَحِدَة ), or simply the Emirates ( ar, الِْإمَارَات ), is a country in Western Asia (The Middle East). It is located at th ...
came under scrutiny, as Denmark
)
, song = ( en, "King Christian stood by the lofty mast")
, song_type = National and royal anthem
, image_map = EU-Denmark.svg
, map_caption =
, subdivision_type = Sovereign state
, subdivision_name = Kingdom of Denmark
, establish ...
suspended the Emirati flights for five days. The European nation said that it barred the flights from the UAE due to growing suspicion of irregularities in the testing process being followed in the Gulf nation. Denmark's Minister of Transport, Benny Engelbrecht
Benny Engelbrecht (born 4 August 1970) is a Danish politician who has been a member of the Folketing for the Social Democrats since the 2007 general elections. He served as the Minister of Transport from 2019 to 2022. He previously served as Min ...
said that they were taking time to ensure that the negative tests of travelers from the Emirates were a real screening carried out appropriately.
United States
New York State
New York State's control measures consisted of PCR tests, stay-at-home measures and strengthening the healthcare system. On 29 February before its first case, the state allowed testing at the Wordsworth Center. They managed to convince the CDC to approve tests at state laboratories and the FDA to approve a test kit. As of 13 March the state was conducting more than 1,000 daily tests, growing to 10,000/day on 19 March. In April, the number exceeded 20,000. Many people queued at hospitals to get tested. On 21 March New York City health officials directed medical providers to test only those entering the hospital, for lack of PPE.
USS ''Theodore Roosevelt''
Following an outbreak, 94% of the 4,800 aircraft carrier crew were tested. Roughly 60 percent of the 600-plus sailors who tested positive were asymptomatic. Five infected sailors who completed quarantine subsequently developed flu-like symptoms and again tested positive.
Nevada
In 2020, Nevada received a donation of 250,000 Covid testing kits, which were a product of China's leading genetics company, BGI Group
BGI Group, formerly Beijing Genomics Institute, is a Chinese genomics company with headquarters in Yantian District, Shenzhen. The company was originally formed in 1999 as a genetics research center to participate in the Human Genome Project. It ...
. A UAE-based firm owned by Tahnoun bin Zayed Al Nahyan, Group 42
Group 42 ( cs, Skupina 42) was a Czech artistic group officially established in 1942 (although its roots date to 1938–1939, forming in 1940). The group's activity ceased in 1948, but its influence on Czech literature and Czech art was still ...
partnered with the BGI Group to supply the testing kits to Nevada
Nevada ( ; ) is a state in the Western region of the United States. It is bordered by Oregon to the northwest, Idaho to the northeast, California to the west, Arizona to the southeast, and Utah to the east. Nevada is the 7th-most extensive, ...
. However, the US Department of Homeland Security
The United States Department of Homeland Security (DHS) is the U.S. federal executive department responsible for public security, roughly comparable to the interior or home ministries of other countries. Its stated missions involve anti-terr ...
and the State Department raised a warning for Nevada hospitals to not use the Chinese-made testing kits, as there were concerns around the involvement of the Chinese government, test accuracy and privacy of the patients.
Delayed testing
A shortage of trained medical laboratory scientist
A medical laboratory scientist (MLS) or clinical laboratory scientist (CLS) or medical technologist (MT) performs diagnostic testing of blood and body fluids in clinical laboratories. The scope of a medical laboratory scientist's work begins wit ...
s, assay reagents, analyzers, transport medium, and PPE coupled with high demand had limited initially limited the availability of testing and led to significantly increased turnaround time
Turnaround time (TAT) is the amount of time taken to complete a process or fulfill a request. The concept thus overlaps with lead time and can be contrasted with cycle time.
Meaning in computing
In computing, turnaround time is the total time t ...
s.
Testing statistics by country
Testing strategies vary by country and over time, with some countries testing very widely,[ The country that tests only people showing symptoms will have a higher figure for "Confirmed"/"tested" than the country that also tests others.]
See also
* 2002–2004 SARS outbreak
The 2002–2004 outbreak of SARS, caused by severe acute respiratory syndrome coronavirus (SARS-CoV or SARS-CoV-1), infected over 8,000 people from 29 countries and territories, and resulted in at least 774 deaths worldwide.
The outbreak was ...
* Coronavirus breathalyzer
A coronavirus breathalyzer is a diagnostic medical device enabling the user to test with 90% or greater accuracy the presence of severe acute respiratory syndrome coronavirus 2 in an exhaled breath.Anthes, E."A Covid Test as Easy as Breathing" Th ...
* Coronavirus disease 2019
*
* COVID-19 pandemic
The COVID-19 pandemic, also known as the coronavirus pandemic, is an ongoing global pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The novel virus was first identi ...
*
References
*
Further reading
*
*
*
External links
* – International testing statistics updated twice a week.
*
''COVID-19 Testing (at least) – now Free for all?'' (CDC; US Congress; CSPAN video/6:00; 12 March 2020)
*
*
*
*
{{Portal bar , COVID-19 , Medicine , Viruses
 COVID-19 testing involves analyzing samples to assess the current or past presence of
COVID-19 testing involves analyzing samples to assess the current or past presence of  Positive viral tests indicate a current infection, while positive antibody tests indicate a prior infection. Other techniques include a CT scan, checking for elevated body temperature, checking for low blood oxygen level, and detection by trained dogs.
Positive viral tests indicate a current infection, while positive antibody tests indicate a prior infection. Other techniques include a CT scan, checking for elevated body temperature, checking for low blood oxygen level, and detection by trained dogs.


 An
An  In Hong Kong test subjects can stay home and receive a specimen tube. They spit into it, return it and later get the result.
In Hong Kong test subjects can stay home and receive a specimen tube. They spit into it, return it and later get the result.
 Economist Paul Romer reported that the US has the technical capacity to scale up to 20 million tests per day, which is his estimate of the scale needed to fully remobilize the economy. The Edmond J. Safra Center for Ethics estimated on 4 April 2020 that this capacity could be available by late July 2020. Romer pointed to single-molecule real-time sequencing equipment from
Economist Paul Romer reported that the US has the technical capacity to scale up to 20 million tests per day, which is his estimate of the scale needed to fully remobilize the economy. The Edmond J. Safra Center for Ethics estimated on 4 April 2020 that this capacity could be available by late July 2020. Romer pointed to single-molecule real-time sequencing equipment from  Countries around the world developed tests independently and in partnership with others.
Countries around the world developed tests independently and in partnership with others.
 Antigen tests are readily available worldwide and have been approved by several health regulators.
Quidel's "Sofia2 SARS Antigen FIA" is a
Antigen tests are readily available worldwide and have been approved by several health regulators.
Quidel's "Sofia2 SARS Antigen FIA" is a  RT-PCR is the most commonly-used diagnostic test. PCR tests by nasopharyngeal swab have a sensitivity of 73%, but systematic analysis of specificity has not been determined due to the lack of PCR studies with a control group.
In one study sensitivity was highest at week one (100%), followed by 89.3%, 66.1%, 32.1%, 5.4% and zero by week six since symptom onset.
Sensitivity is also a function of the number of PCR cycles, as well as time and temperature between sample collection and analysis. A cycle threshold of 20 cycles would be adequate to detect SARS-Cov-2 in a highly infective person. Cycle thresholds above 34 are increasingly likely to give false positives outside of high biosafety level facilities.
On July 16, 2020,
RT-PCR is the most commonly-used diagnostic test. PCR tests by nasopharyngeal swab have a sensitivity of 73%, but systematic analysis of specificity has not been determined due to the lack of PCR studies with a control group.
In one study sensitivity was highest at week one (100%), followed by 89.3%, 66.1%, 32.1%, 5.4% and zero by week six since symptom onset.
Sensitivity is also a function of the number of PCR cycles, as well as time and temperature between sample collection and analysis. A cycle threshold of 20 cycles would be adequate to detect SARS-Cov-2 in a highly infective person. Cycle thresholds above 34 are increasingly likely to give false positives outside of high biosafety level facilities.
On July 16, 2020,