|
Toothpaste
Toothpaste is a paste or gel dentifrice that is used with a toothbrush to clean and maintain the aesthetics of Human tooth, teeth. Toothpaste is used to promote oral hygiene: it is an abrasive that aids in removing dental plaque and food from the teeth, assists in suppressing halitosis, and delivers active ingredients (most commonly fluoride) to help prevent tooth decay (dental caries) and gum disease (gingivitis).American Dental Association Description of Toothpaste Due to variations in composition and fluoride content, not all toothpastes are equally effective in maintaining oral health. The decline of tooth decay during the 20th century has been attributed to the introduction and regular use of fluoride-containing toothpastes worldwide. Large amounts of swallowed toothpaste can be poisonous. Common colors for toothpaste include white (sometimes with colored stripes or green tint) and blue. History Early toothpastes Since 5000 BCE, the Egyptians made a tooth powder, which cons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Tooth Decay
Tooth decay, also known as caries,The word 'caries' is a mass noun, and is not a plural of 'carie'.'' is the breakdown of teeth due to acids produced by bacteria. The resulting cavities may be a number of different colors, from yellow to black. Symptoms may include pain and difficulty eating. Complications may include periodontal disease, inflammation of the tissue around the tooth, tooth loss and infection or dental abscess, abscess formation. Tooth regeneration is an ongoing Stem-cell therapy, stem cell–based field of study that aims to find methods to reverse the effects of decay; current methods are based on easing symptoms. The cause of cavities is acid from bacteria dissolving the hard tissues of the teeth (Tooth enamel, enamel, dentin and cementum). The acid is produced by the bacteria when they break down food debris or sugar on the tooth surface. Simple sugars in food are these bacteria's primary energy source and thus a diet high in simple sugar is a risk factor. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Oral Hygiene
Oral hygiene is the practice of keeping one's oral cavity clean and free of disease and other problems (e.g. bad breath) by regular brushing of the teeth (dental hygiene) and adopting good hygiene habits. It is important that oral hygiene be carried out on a regular basis to enable prevention of dental disease and bad breath. The most common types of dental disease are tooth decay (''cavities'', ''dental caries'') and gum diseases, including gingivitis, and periodontitis. General guidelines for adults suggest brushing at least twice a day with a fluoridated toothpaste: brushing before going to sleep at night and after breakfast in the morning. Cleaning between the teeth is called interdental cleaning and is as important as tooth brushing. This is because a toothbrush cannot reach between the teeth and therefore only removes about 50% of plaque from the surface of the teeth. There are many tools available for interdental cleaning which include Dental floss, floss, tape and interden ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hydroxyapatite
Hydroxyapatite (International Mineralogical Association, IMA name: hydroxylapatite) (Hap, HAp, or HA) is a naturally occurring mineral form of calcium apatite with the Chemical formula, formula , often written to denote that the Crystal structure, crystal unit cell comprises two entities. It is the Hydroxy group, hydroxyl endmember of the complex apatite, apatite group. The ion can be replaced by fluorine, fluoride or chlorine, chloride, producing fluorapatite or chlorapatite. It crystallizes in the hexagonal (crystal system), hexagonal crystal system. Pure hydroxyapatite powder is white. Naturally occurring apatites can, however, also have brown, yellow, or green colorations, comparable to the discolorations of dental fluorosis. Up to 50% by volume and 70% by weight of human bone is a modified form of hydroxyapatite, known as bone mineral. Carbonated calcium-deficient hydroxyapatite is the main mineral of which dental enamel and dentin are composed. Hydroxyapatite crystals a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an Inorganic chemistry, inorganic, Monatomic ion, monatomic Ion#Anions and cations, anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important Reagent, chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin. Fluoride is the simplest fluorine anion. In terms of charge and size, the fluoride ion resembles the hydroxide ion. Fluoride ions occur on Earth in several minerals, particularly fluorite, but are present only in trace quantities in bodies of water in nature. Nomenclature Fluorides include compounds that contain ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Toothbrush
A toothbrush is a special type of brush used to clean the Human tooth, teeth, gingiva, gums, and tongue. It consists of a head of tightly clustered bristles, atop of which toothpaste can be applied, mounted on a handle (grip), handle which facilitates the cleaning of hard-to-reach areas of the mouth. They should be used in conjunction with something to clean between the teeth where the bristles of the toothbrush cannot reach - for example Dental floss, floss, tape, interdental brushes or toothpicks. They are available with different bristle textures, sizes, and forms. Most dentists recommend using a soft toothbrush since hard-bristled toothbrushes can damage tooth enamel and irritate the gums. Because many common and effective ingredients in toothpaste are harmful if swallowed in large doses, tooth paste should instead be spat out. The act of brushing teeth is most often done at a sink within the kitchen or bathroom, where the brush may be rinsed off afterwards to remove any deb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Dentifrice
Dentifrices, including toothpowder and toothpaste, are agents used along with a toothbrush to aid in removal of dental plaque. They are supplied in paste, powder or gel. Many dentifrices have been produced over the years, some focusing on marketing strategies to sell products, such as offering tooth whitening, whitening capabilities. The most essential dentifrice recommended by dentists is toothpaste which is used in conjunction with a toothbrush to help remove food debris and dental plaque. Dentifrice is also the French language, French word for toothpaste. Types Toothpaste Toothpaste is a dentifrice used in conjunction with a toothbrush to help maintain oral hygiene. The essential components are an abrasive, binder, surfactant and humectant. Other ingredients are also used. The main purpose of the paste is to help remove debris and plaque with some marketed to serve accessory functions such as breath freshening and teeth whitening. Tooth powder Tooth powder was histo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
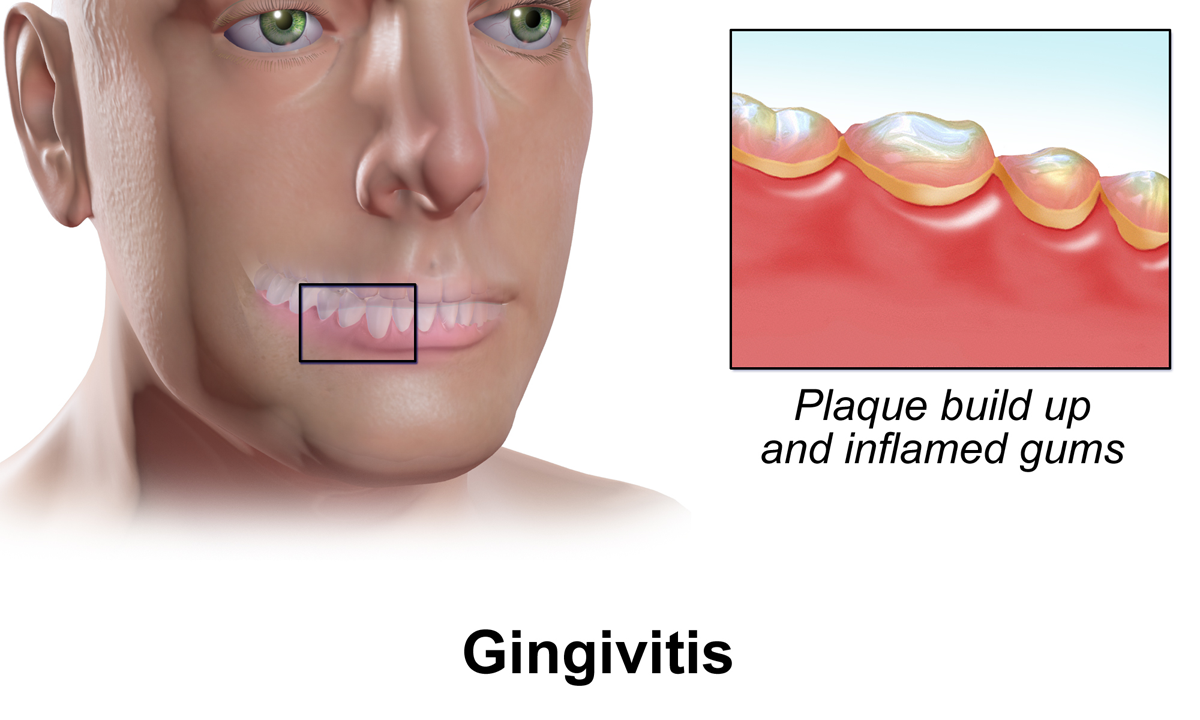
Gingivitis
Gingivitis is a non-destructive disease that causes inflammation of the gums; ulitis is an alternative term. The most common form of gingivitis, and the most common form of periodontal disease overall, is in response to bacterial biofilms (also called plaque) that are attached to tooth surfaces, termed ''plaque-induced gingivitis''. Most forms of gingivitis are plaque-induced. While some cases of gingivitis never progress to periodontitis, periodontitis is always preceded by gingivitis. Gingivitis is reversible with good oral hygiene; however, without treatment, gingivitis can progress to periodontitis, in which the inflammation of the gums results in tissue destruction and bone resorption around the teeth. Periodontitis can ultimately lead to tooth loss. Signs and symptoms The symptoms of gingivitis are somewhat non-specific and manifest in the gum tissue as the classic signs of inflammation: *Swollen gums *Bright red gums *Gums that are tender or painful to the touch *B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Calcium Hydrogen Phosphate
Dicalcium phosphate is the calcium phosphate with the formula CaHPO4 and its dihydrate. The "di" prefix in the common name arises because the formation of the HPO42– anion involves the removal of two protons from phosphoric acid, H3PO4. It is also known as dibasic calcium phosphate or calcium monohydrogen phosphate. Dicalcium phosphate is used as a food additive, and it is found in some toothpastes as a polishing agent and biomaterial. Preparation Dibasic calcium phosphate is produced by neutralizing calcium hydroxide with phosphoric acid, precipitating the dihydrate as a solid. At 60 °C the anhydrous form is precipitated: To prevent degradation that would form hydroxyapatite, sodium pyrophosphate or magnesium phosphate tribasic, trimagnesium phosphate octahydrate are added when, for example, dibasic calcium phosphate dihydrate is to be used as a polishing agent in toothpaste. In a continuous process calcium chloride, CaCl2 can be treated with diammonium phosphate, (NH4) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Pumice
Pumice (), called pumicite in its powdered or dust form, is a volcanic rock that consists of extremely vesicular rough-textured volcanic glass, which may or may not contain crystals. It is typically light-colored. Scoria is another vesicular volcanic rock that differs from pumice in having larger vesicles, thicker vesicle walls, and being dark colored and denser.Jackson, J.A., J. Mehl, and K. Neuendorf (2005) ''Glossary of Geology'' American Geological Institute, Alexandria, Virginia. 800 pp. McPhie, J., M. Doyle, and R. Allen (1993) ''Volcanic Textures A guide to the interpretation of textures in volcanic rocks'' Centre for Ore Deposit and Exploration Studies, University of Tasmania, Hobart, Tasmania..198 pp. Pumice is created when super-heated, highly pressurized rock is rapidly ejected from a volcano. The unusual foamy configuration of pumice happens because of simultaneous rapid cooling and rapid depressurization. The depressurization creates bubbles by lowering the sol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Miswak
The miswak is a teeth-cleaning twig made from the '' Salvadora persica'' tree. The miswak's properties have been described thus: "Apart from their antibacterial activity which may help control the formation and activity of dental plaque, they can be used effectively as a natural toothbrush for teeth cleaning. Such sticks are effective, inexpensive, common, available, and contain many medical properties". The ''miswak'' is predominant in Muslim-inhabited areas. It is commonly used in the Arabian Peninsula, the Horn of Africa, North Africa, parts of the Sahel, the Indian subcontinent, and Central Asia. Science The World Health Organization (WHO) recommended the use of the ''miswak'' in 1986, but in 2000, an international consensus report on oral hygiene concluded that further research was needed to document the effect of the ''miswak''. Some of this further research has been done on a population of 203, and concluded, "that the periodontal status of miswak users in this Sudane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ziryab
Abu al-Hasan 'Ali Ibn Nafi (; 789– 857), commonly known as Ziryab, was a singer, oud and lute player, composer, poet, and teacher. He lived and worked in what is now Iraq, Northern Africa and Andalusia during the medieval Islamic period. He was also a polymath, with knowledge in astronomy, geography, meteorology, botanics, cosmetics, culinary art, and fashion. His nickname, "Ziryab", comes from the Persian and Kurdish word for jay-bird , pronounced "Zaryāb". He was also known as ('blackbird') in Spanish. He was active at the Umayyad court of Córdoba in Islamic Iberia. He first achieved fame at the Abbasid court in Baghdad, his birthplace, as a performer and student of the musician and composer Ibrahim al-Mawsili. Ziryab was a gifted pupil of Ibrahim al-Mawsili in Baghdad, where he got his beginner lessons. He left Baghdad during the reign of the Abbasid caliph al-Ma'mun and moved to Córdoba, where he was accepted as a court musician in the court of Abd ar-Rahman ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |