|
Phenyl
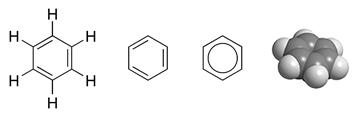
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. Phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring. Nomenclature Usually, a "phenyl group" is synonymous with C6H5− and is represented by the symbol Ph or, archaically, Φ. Benzene is sometimes denoted as PhH. Phenyl groups are generally attached to other atoms or groups. For example, triphenylmethane (Ph3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenyl Radical Group
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. Phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring. Nomenclature Usually, a "phenyl group" is synonymous with C6H5− and is represented by the symbol Ph or, archaically, Φ. Benzene is sometimes denoted as PhH. Phenyl groups are generally attached to other atoms or groups. For example, triphenylmethane (Ph3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenyllithium
Phenyllithium or lithobenzene is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses. Crystalline phenyllithium is colorless; however, solutions of phenyllithium are various shades of brown or red depending on the solvent used and the impurities present in the solute. Preparation Phenyllithium was first produced by the reaction of lithium metal with diphenylmercury: :(C6Η5)2Ηg + 2Li → 2C6Η5Li + Ηg Reaction of a phenyl halide with lithium metal produces phenyllithium: :X-Ph + 2Li → Ph-Li + LiX Phenyllithium can also be synthesized with a metal-halogen exchange reaction: :n-BuLi + X-Ph → n-BuX + Ph-Li The predominant method of producing phenyllithium today are the latter two syntheses. Reactions The primary use of PhLi is to facilitate formation of carbon-carbon bonds by nucleophilic addition and substitut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. Fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylmagnesium Bromide
Phenylmagnesium bromide, with the simplified formula , is a magnesium-containing organometallic compound. It is commercially available as a solution in diethyl ether or tetrahydrofuran (THF). Phenylmagnesium bromide is a Grignard reagent. It is often used as a synthetic equivalent for the phenyl "Ph−" synthon. Preparation Phenylmagnesium bromide is commercially available as solutions of diethyl ether or THF. Laboratory preparation involves treating bromobenzene with magnesium metal, usually in the form of turnings. A small amount of iodine may be used to activate the magnesium to initiate the reaction. Coordinating solvents such as ether or THF, are required to solvate (complex) the magnesium(II) center. The solvent must be aprotic since alcohols and water contain an acidic proton and thus react with phenylmagnesium bromide to give benzene. Carbonyl-containing solvents, such as acetone and ethyl acetate, are also incompatible with the reagent. Structure Although phenylmagn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorobenzene
Chlorobenzene is an aromatic organic compound with the chemical formula C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals. Uses Historical The major use of chlorobenzene is as an intermediate in the production of herbicides, dyestuffs, and rubber. Chlorobenzene is also used as a high-boiling solvent in industrial applications as well as in the laboratory. Chlorobenzene is nitrated on a large scale to give a mixture of 2-nitrochlorobenzene and 4-nitrochlorobenzene, which are separated. These mononitrochlorobenzenes are converted to related 2-nitrophenol, 2-nitroanisole, bis(2-nitrophenyl)disulfide, and 2-nitroaniline by nucleophilic displacement of the chloride, with respectively sodium hydroxide, sodium methoxide, sodium disulfide, and ammonia. The conversions of the 4-nitro derivative are similar. Chlorobenzene once was used in the manufacture of pesticides, most notably DDT, by reaction with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylmethane
Triphenylmethane, or triphenyl methane, is the hydrocarbon with the formula (C6H5)3CH. This colorless solid is soluble in nonpolar organic solvents and not in water. Triphenylmethane is the basic skeleton of many synthetic dyes called triarylmethane dyes, many of them are pH indicators, and some display fluorescence. A trityl group in organic chemistry is a triphenylmethyl group Ph3C, e.g. triphenylmethyl chloride (trityl chloride) and the triphenylmethyl radical (trityl radical). Preparation Triphenylmethane was first synthesized in 1872 by the German chemist August Kekulé and his Belgian student Antoine Paul Nicolas Franchimont (1844–1919) by heating diphenylmercury (Hg(C6H5)2, ''Quecksilberdiphenyl'') with benzal chloride (C6H5CHCl2, ''Benzylenchlorid''). Triphenylmethane can be synthesized by Friedel–Crafts reaction from benzene and chloroform with aluminium chloride catalyst: :3 C6H6 + CHCl3 → Ph3CH + 3 HCl Alternatively, benzene may react with carbon tetrachloride us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structure, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. History Discovery The word "''benzene''" derives from "''gum benzoin''" (benzoin res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, or unsaturated, like hexene and hexyne. Open-chain compounds, whether straight or branched, and which contain no rings of any type, are always aliphatic. Cyclic compounds can be aliphatic if they are not aromatic. Structure Aliphatic compounds can be saturated, joined by single bonds (alkanes), or unsaturated, with double bonds (alkenes) or triple bonds (alkynes). If other elements (heteroatoms) are bound to the carbon chain, the most common being oxygen, nitrogen, sulfur, and chlorine, it is no longer a hydrocarbon, and therefore no longer an aliphatic compound. The least complex aliphatic compound is methane (CH4). Properties Most aliphatic compounds are flammable, allowing the use of hydrocarbons as fuel, such as methane in Buns ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Orbital Theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century. In molecular orbital theory, electrons in a molecule are not assigned to individual chemical bonds between atoms, but are treated as moving under the influence of the atomic nuclei in the whole molecule. Quantum mechanics describes the spatial and energetic properties of electrons as molecular orbitals that surround two or more atoms in a molecule and contain valence electrons between atoms. Molecular orbital theory revolutionized the study of chemical bonding by approximating the states of bonded electrons—the molecular orbitals—as linear combinations of atomic orbitals (LCAO). These approximations are made by applying the density functional theory (DFT) or Hartree–Fock (HF) models to the Schrödinger equation. Molecular orbital theory and valence bond theory are the foundational ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ångström
The angstromEntry "angstrom" in the Oxford online dictionary. Retrieved on 2019-03-02 from https://en.oxforddictionaries.com/definition/angstrom.Entry "angstrom" in the Merriam-Webster online dictionary. Retrieved on 2019-03-02 from https://www.merriam-webster.com/dictionary/angstrom. (, ; , ) or ångström is a metric unit of length equal to m; that is, one ten-billionth ( US) of a metre, a hundred-millionth of a centimetre,Entry "angstrom" in the Oxford English Dictionary, 2nd edition (1986). Retrieved on 2021-11-22 from https://www.oed.com/oed2/00008552. 0.1 nanometre, or 100 picometres. Its symbol is Å, a letter of the Swedish alphabet. The unit is named after the Swedish physicist Anders Jonas Ångström (1814–1874). The angstrom is often used in the natural sciences and technology to express sizes of atoms, molecules, microscopic biological structures, and lengths of chemical bonds, arrangement of atoms in crystals,Arturas Vailionis (2015):Geometry of Crystals Lect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Ring Current
An aromatic ring current is an effect observed in aromatic molecules such as benzene and naphthalene. If a magnetic field is directed perpendicular to the plane of the aromatic system, a ring current is induced in the delocalized π electrons of the aromatic ring. This is a direct consequence of Ampère's law; since the electrons involved are free to circulate, rather than being localized in bonds as they would be in most non-aromatic molecules, they respond much more strongly to the magnetic field. The ring current creates its own magnetic field. Outside the ring, this field is in the same direction as the externally applied magnetic field; inside the ring, the field counteracts the externally applied field. As a result, the net magnetic field outside the ring is greater than the externally applied field alone, and is less inside the ring. Aromatic ring currents are relevant to NMR spectroscopy, as they dramatically influence the chemical shifts of 1H nuclei ("protons") in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Magnetic Resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crystalline materials. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



2.png)