|
Human Body
The human body is the entire structure of a Human, human being. It is composed of many different types of Cell (biology), cells that together create Tissue (biology), tissues and subsequently Organ (biology), organs and then Organ system, organ systems. The external human body consists of a human head, head, hair, neck, torso (which includes the thorax and abdomen), Sex organ, genitals, arms, Hand, hands, human leg, legs, and Foot, feet. The internal human body includes organs, Human tooth, teeth, bones, muscle, tendons, ligaments, blood vessels and blood, lymphatic vessels and lymph. The study of the human body includes anatomy, physiology, histology and embryology. The body Anatomical variation, varies anatomically in known ways. Physiology focuses on the systems and organs of the human body and their functions. Many systems and mechanisms interact in order to maintain homeostasis, with safe levels of substances such as sugar, iron, and oxygen in the blood. The body is st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Human Body 02
Humans (''Homo sapiens'') or modern humans are the most common and widespread species of primate, and the last surviving species of the genus ''Homo''. They are Hominidae, great apes characterized by their Prehistory of nakedness and clothing#Evolution of hairlessness, hairlessness, bipedality, bipedalism, and high Human intelligence, intelligence. Humans have large Human brain, brains, enabling more advanced cognitive skills that facilitate successful adaptation to varied environments, development of sophisticated tools, and formation of complex social structures and civilizations. Humans are Sociality, highly social, with individual humans tending to belong to a Level of analysis, multi-layered network of distinct social groups — from families and peer groups to corporations and State (polity), political states. As such, social interactions between humans have established a wide variety of Value theory, values, norm (sociology), social norms, languages, and traditions (co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bone
A bone is a rigid organ that constitutes part of the skeleton in most vertebrate animals. Bones protect the various other organs of the body, produce red and white blood cells, store minerals, provide structure and support for the body, and enable mobility. Bones come in a variety of shapes and sizes and have complex internal and external structures. They are lightweight yet strong and hard and serve multiple functions. Bone tissue (osseous tissue), which is also called bone in the uncountable sense of that word, is hard tissue, a type of specialised connective tissue. It has a honeycomb-like matrix internally, which helps to give the bone rigidity. Bone tissue is made up of different types of bone cells. Osteoblasts and osteocytes are involved in the formation and mineralisation of bone; osteoclasts are involved in the resorption of bone tissue. Modified (flattened) osteoblasts become the lining cells that form a protective layer on the bone surface. The mine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homeostasis
In biology, homeostasis (British English, British also homoeostasis; ) is the state of steady internal physics, physical and chemistry, chemical conditions maintained by organism, living systems. This is the condition of optimal functioning for the organism and includes many variables, such as body temperature and fluid balance, being kept within certain pre-set limits (homeostatic range). Other variables include the pH of extracellular fluid, the concentrations of sodium, potassium, and calcium ions, as well as the blood sugar level, and these need to be regulated despite changes in the environment, diet, or level of activity. Each of these variables is controlled by one or more regulators or homeostatic mechanisms, which together maintain life. Homeostasis is brought about by a natural resistance to change when already in optimal conditions, and equilibrium is maintained by many regulatory mechanisms; it is thought to be the central motivation for all organic action. All home ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anatomical Variation
An anatomical variation, anatomical variant, or anatomical variability is a presentation of body structure with Morphology (biology), morphological features different from those that are typically described in the majority of individuals. Anatomical variations are categorized into three types including morphometric (size or shape), consistency (present or absent), and spatial (proximal/distal or right/left). Variations are seen as normal in the sense that they are found consistently among different individuals, are mostly without symptoms, and are termed anatomical variations rather than abnormalities. Anatomical variations are mainly caused by genetics and may vary considerably between different populations. The rate of variation considerably differs between single organ (anatomy), organs, particularly in muscles. Knowledge of anatomical variations is important in order to distinguish them from pathological conditions. A very early paper published in 1898, presented anatomic var ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Embryology
Embryology (from Ancient Greek, Greek ἔμβρυον, ''embryon'', "the unborn, embryo"; and -λογία, ''-logy, -logia'') is the branch of animal biology that studies the Prenatal development (biology), prenatal development of gametes (sex cells), fertilization, and development of embryos and fetuses. Additionally, embryology encompasses the study of congenital disorders that occur before birth, known as teratology. Early embryology was proposed by Marcello Malpighi, and known as preformationism, the theory that organisms develop from pre-existing miniature versions of themselves. Aristotle proposed the theory that is now accepted, Epigenesis (biology), epigenesis. Epigenesis (biology), Epigenesis is the idea that organisms develop from seed or egg in a sequence of steps. Modern embryology developed from the work of Karl Ernst von Baer, though accurate observations had been made in Italy by anatomists such as Aldrovandi and Leonardo da Vinci in the Renaissance. Comparative ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
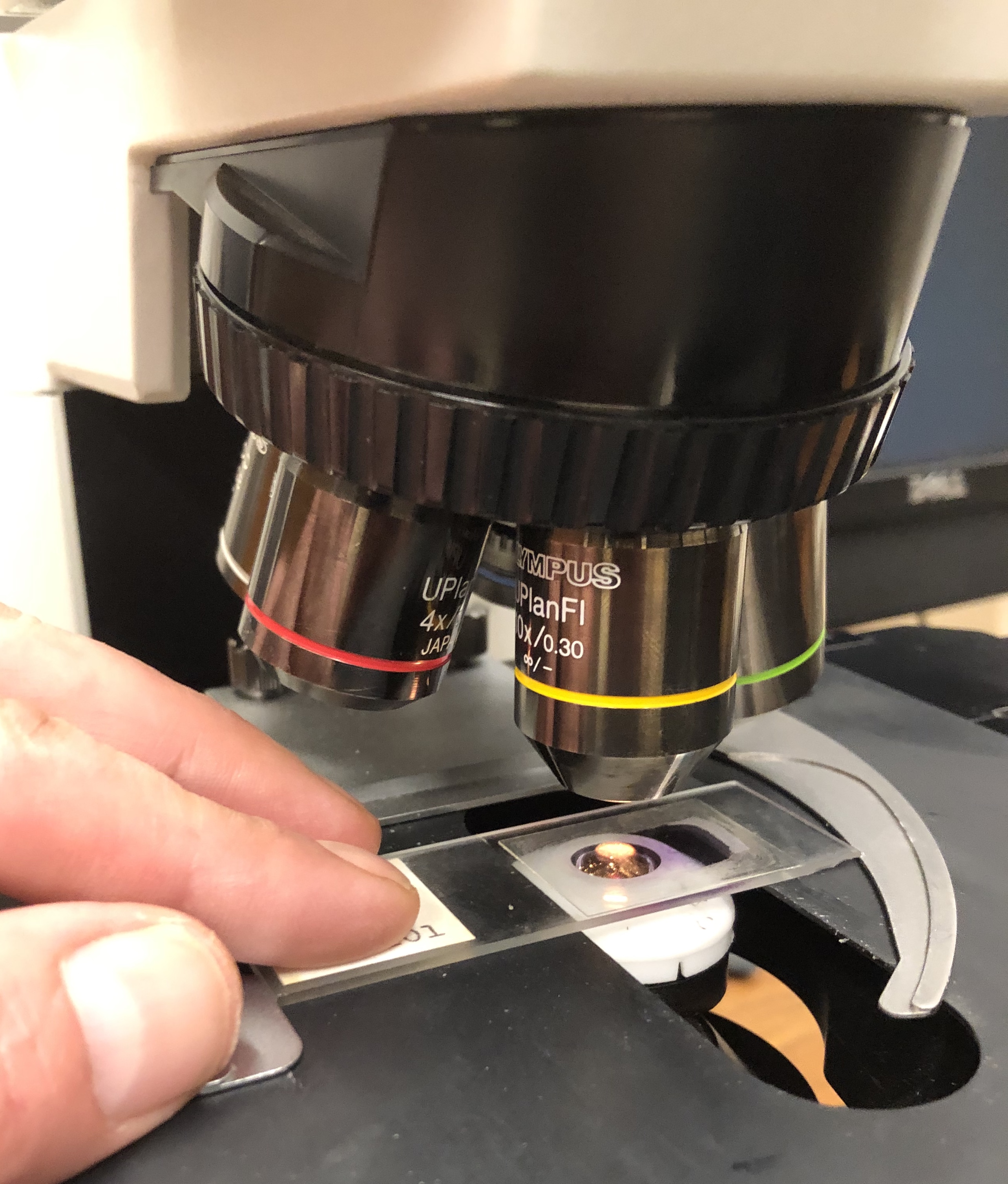
Histology
Histology, also known as microscopic anatomy or microanatomy, is the branch of biology that studies the microscopic anatomy of biological tissue (biology), tissues. Histology is the microscopic counterpart to gross anatomy, which looks at larger structures visible without a microscope. Although one may divide microscopic anatomy into ''organology'', the study of organs, ''histology'', the study of tissues, and ''cytology'', the study of cell (biology), cells, modern usage places all of these topics under the field of histology. In medicine, histopathology is the branch of histology that includes the microscopic identification and study of diseased tissue. In the field of paleontology, the term paleohistology refers to the histology of fossil organisms. Biological tissues Animal tissue classification There are four basic types of animal tissues: muscle tissue, nervous tissue, connective tissue, and epithelial tissue. All animal tissues are considered to be subtypes of these ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physiology
Physiology (; ) is the science, scientific study of function (biology), functions and mechanism (biology), mechanisms in a life, living system. As a branches of science, subdiscipline of biology, physiology focuses on how organisms, organ systems, individual organ (biology), organs, cell (biology), cells, and biomolecules carry out chemistry, chemical and physics, physical functions in a living system. According to the classes of organisms, the field can be divided into clinical physiology, medical physiology, Zoology#Physiology, animal physiology, plant physiology, cell physiology, and comparative physiology. Central to physiological functioning are biophysics, biophysical and biochemical processes, homeostasis, homeostatic control mechanisms, and cell signaling, communication between cells. ''Physiological state'' is the condition of normal function. In contrast, ''pathology, pathological state'' refers to abnormality (behavior), abnormal conditions, including human diseases. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anatomy
Anatomy () is the branch of morphology concerned with the study of the internal structure of organisms and their parts. Anatomy is a branch of natural science that deals with the structural organization of living things. It is an old science, having its beginnings in prehistoric times. Anatomy is inherently tied to developmental biology, embryology, comparative anatomy, evolutionary biology, and phylogeny, as these are the processes by which anatomy is generated, both over immediate and long-term timescales. Anatomy and physiology, which study the structure and function of organisms and their parts respectively, make a natural pair of related disciplines, and are often studied together. Human anatomy is one of the essential basic sciences that are applied in medicine, and is often studied alongside physiology. Anatomy is a complex and dynamic field that is constantly evolving as discoveries are made. In recent years, there has been a significant increase in the use of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lymph
Lymph () is the fluid that flows through the lymphatic system, a system composed of lymph vessels (channels) and intervening lymph nodes whose function, like the venous system, is to return fluid from the tissues to be recirculated. At the origin of the fluid-return process, interstitial fluid—the fluid between the cells in all body tissues—enters the lymph capillaries. This lymphatic fluid is then transported via progressively larger lymphatic vessels through lymph nodes, where substances are removed by tissue lymphocytes and circulating lymphocytes are added to the fluid, before emptying ultimately into the right or the left subclavian vein, where it mixes with central venous blood. Because it is derived from interstitial fluid, with which blood and surrounding cells continually exchange substances, lymph undergoes continual change in composition. It is generally similar to blood plasma, which is the fluid component of blood. Lymph returns proteins and excess interstiti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lymphatic Vessel
The lymphatic vessels (or lymph vessels or lymphatics) are thin-walled vessels (tubes), structured like blood vessels, that carry lymph. As part of the lymphatic system, lymph vessels are complementary to the cardiovascular system. Lymph vessels are lined by endothelium, endothelial cells, and have a thin layer of smooth muscle, and adventitia that binds the lymph vessels to the surrounding tissue. Lymph vessels are devoted to the propulsion of the lymph from the lymph capillaries, which are mainly concerned with the absorption of interstitial fluid from the tissues. Lymph capillaries are slightly bigger than their counterpart capillary, capillaries of the vascular system. Lymph vessels that carry lymph to a lymph node are called afferent lymph vessels, and those that carry it from a lymph node are called efferent lymph vessels, from where the lymph may travel to another lymph node, may be returned to a vein, or may travel to a larger lymph duct. Lymph ducts drain the lymph into ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells. Blood is composed of blood cells suspended in blood plasma. Plasma, which constitutes 55% of blood fluid, is mostly water (92% by volume), and contains proteins, glucose, mineral ions, and hormones. The blood cells are mainly red blood cells (erythrocytes), white blood cells (leukocytes), and (in mammals) platelets (thrombocytes). The most abundant cells are red blood cells. These contain hemoglobin, which facilitates oxygen transport by reversibly binding to it, increasing its solubility. Jawed vertebrates have an adaptive immune system, based largely on white blood cells. White blood cells help to resist infections and parasites. Platelets are important in the clotting of blood. Blood is circulated around the body through blood vessels by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood Vessel
Blood vessels are the tubular structures of a circulatory system that transport blood throughout many Animal, animals’ bodies. Blood vessels transport blood cells, nutrients, and oxygen to most of the Tissue (biology), tissues of a Body (biology), body. They also take waste and carbon dioxide away from the tissues. Some tissues such as cartilage, epithelium, and the lens (anatomy), lens and cornea of the eye are not supplied with blood vessels and are termed ''avascular''. There are five types of blood vessels: the arteries, which carry the blood away from the heart; the arterioles; the capillaries, where the exchange of water and chemicals between the blood and the tissues occurs; the venules; and the veins, which carry blood from the capillaries back towards the heart. The word ''vascular'', is derived from the Latin ''vas'', meaning ''vessel'', and is mostly used in relation to blood vessels. Etymology * artery – late Middle English; from Latin ''arteria'', from Gree ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |