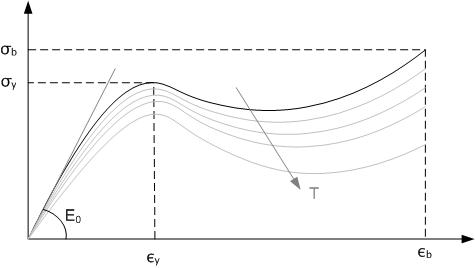
|
Dyneema
Ultra-high-molecular-weight polyethylene (UHMWPE, UHMW) is a subset of the thermoplastic polyethylene. Also known as high-modulus polyethylene (HMPE), it has extremely long chains, with a molecular mass typically between 2 and 6 million amu. The longer chain serves to transfer load more effectively to the polymer backbone by strengthening intermolecular interactions. This results in a very tough material, with the highest impact strength of any thermoplastic presently made. UHMWPE is odorless, tasteless, and nontoxic. It embodies all the characteristics of high-density polyethylene (HDPE) with the added traits of being resistant to concentrated acids and alkalis, as well as numerous organic solvents. It is highly resistant to corrosive chemicals except oxidizing acids; has extremely low moisture absorption and a very low coefficient of friction; is self-lubricating (see boundary lubrication); and is highly resistant to abrasion, in some forms being 15 times more resistant to a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
DSM (company)
Koninklijke DSM N.V. (Royal DSM, commonly known as DSM, which is the acronym for ''Dutch State Mines''), was a Dutch multinational corporation active in the fields of health, nutrition and materials. Headquartered in Maastricht, at the end of 2017 DSM employed 21,054 people in approximately 50 countries and posted net Sales (accounting), sales of €8.632 billion in 2018 and €9.204 billion in 2021. In May 2023, it merged with the Swiss company Firmenich to form a new entity named DSM-Firmenich, dsm-firmenich. History DSM was formed by the Dutch state in 1902 to coal mine, mine coal reserves in southern Limburg (Netherlands), Limburg and although the company had diversified into commodity chemicals and petrochemicals by 1973, when the last mine closed, DSM retains a link to its origins by continuing to use the initials, originally an abbreviation for Dutch State Mines, to this day. During World War II researchers worked on penicillin. The code name Bacinol was used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Thermoplastic
A thermoplastic, or thermosoftening plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling. Most thermoplastics have a high molecular weight. The polymer chains associate by intermolecular forces, which weaken rapidly with increased temperature, yielding a viscous liquid. In this state, thermoplastics may be reshaped, and are typically used to produce parts by various polymer processing techniques such as injection molding, compression molding, calendering, and extrusion. Thermoplastics differ from thermosetting polymers (or "thermosets"), which form irreversible chemical bonds during the curing process. Thermosets do not melt when heated, but typically decompose and do not reform upon cooling. Above its glass transition temperature and below its melting point, the physical properties of a thermoplastic change drastically without an associated phase change. Some thermoplastics do not fully ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ruhrchemie
Ruhrchemie is a chemical company founded in 1927/28. The company, based in the Holten district of Oberhausen, is now the German headquarters of the OQ (company). It is now formally known as OQ Werk Ruhrchemie. History For a short time at the beginning of the 20th century, one of the first German airports seemed to be built in the Holtener Bruch, which had been largely drained by the canalization of the Emscher river. However, it remained with air shows and training flights; the course and outcome of the First World War prevented the airport project from being realized. Instead, the area was used for industrial purposes at the end of the 1920s. The company was founded in 1927 by numerous Ruhr mining companies as :de:Kohlechemie AG. It was renamed Ruhrchemie AG in April 1928 and began producing fertilizers at the Holten site in 1929. In 1936, the first plant for the production of liquid hydrocarbons using the Fischer-Tropsch process went into operation. In 1938, Otto Roelen devel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Crystallinity
Crystallinity refers to the degree of structural order in a solid. In a crystal, the atoms or molecules are arranged in a regular, periodic manner. The degree of crystallinity has a large influence on hardness, density, transparency and diffusion. In an ideal gas, the relative positions of the atoms or molecules are completely random. Amorphous materials, such as liquids and glasses, represent an intermediate case, having order over short distances (a few atomic or molecular spacings) but not over longer distances. Many materials, such as glass-ceramics and some polymers, can be prepared in such a way as to produce a mixture of crystalline and amorphous regions. In such cases, crystallinity is usually specified as a percentage of the volume of the material that is crystalline. Even within materials that are completely crystalline, however, the degree of structural perfection can vary. For instance, most metallic alloys are crystalline, but they usually comprise many independent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Tensile Stress
In continuum mechanics, stress is a physical quantity that describes forces present during deformation. For example, an object being pulled apart, such as a stretched elastic band, is subject to ''tensile'' stress and may undergo elongation. An object being pushed together, such as a crumpled sponge, is subject to ''compressive'' stress and may undergo shortening. The greater the force and the smaller the cross-sectional area of the body on which it acts, the greater the stress. Stress has dimension of force per area, with SI units of newtons per square meter (N/m2) or pascal (Pa). Stress expresses the internal forces that neighbouring particles of a continuous material exert on each other, while ''strain'' is the measure of the relative deformation of the material. For example, when a solid vertical bar is supporting an overhead weight, each particle in the bar pushes on the particles immediately below it. When a liquid is in a closed container under pressure, each pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Van Der Waals Forces
In molecular physics and chemistry, the van der Waals force (sometimes van der Waals' force) is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and therefore more susceptible to disturbance. The van der Waals force quickly vanishes at longer distances between interacting molecules. Named after Dutch physicist Johannes Diderik van der Waals, the van der Waals force plays a fundamental role in fields as diverse as supramolecular chemistry, structural biology, polymer science, nanotechnology, surface science, and condensed matter physics. It also underlies many properties of organic compounds and molecular solids, including their solubility in polar and non-polar media. If no other force is present, the distance between atoms at which the force becomes repulsive rather than attractive as the atoms approach one another is called the va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Polyolefin
A polyolefin is a type of polymer with the general formula (CH2CHR)n where R is an alkyl group. They are usually derived from a small set of simple olefins (alkenes). Dominant in a commercial sense are polyethylene and polypropylene. More specialized polyolefins include Polyisobutene, polyisobutylene and polymethylpentene. They are all colorless or white oils or solids. Many copolymers are known, such as polybutene, which derives from a mixture of different butene isomers. The name of each polyolefin indicates the olefin from which it is prepared; for example, polyethylene is derived from ethylene, and polymethylpentene is derived from 4-methyl-1-pentene. Polyolefins are not olefins themselves because the double bond of each olefin monomer is opened in order to form the polymer. Monomers having more than one double bond such as butadiene and isoprene yield polymers that contain double bonds (polybutadiene and polyisoprene) and are usually not considered polyolefins. Polyolefins are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Polyethylene Repeat Unit
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bottles, cups, jars, etc.). , over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market. Many kinds of polyethylene are known, with most having the chemical formula (C2H4)''n''. PE is usually a mixture of similar polymers of ethylene, with various values of ''n''. It can be ''low-density'' or ''high-density'' and many variations thereof. Its properties can be modified further by crosslinking or copolymerization. All forms are nontoxic as well as chemically resilient, contributing to polyethylene's popularity as a multi-use plastic. However, polyethylene's chemical resilience also makes it a long-lived and decomposition-resistant pollutant when disposed of improperly. Being a h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Vertebral Column
The spinal column, also known as the vertebral column, spine or backbone, is the core part of the axial skeleton in vertebrates. The vertebral column is the defining and eponymous characteristic of the vertebrate. The spinal column is a segmented column of vertebrae that surrounds and protects the spinal cord. The vertebrae are separated by intervertebral discs in a series of cartilaginous joints. The dorsal portion of the spinal column houses the spinal canal, an elongated body cavity, cavity formed by the alignment of the vertebral neural arches that encloses and protects the spinal cord, with spinal nerves exiting via the intervertebral foramina to innervate each body segment. There are around 50,000 species of animals that have a vertebral column. The human spine is one of the most-studied examples, as the general structure of human vertebrae is fairly homology (biology), typical of that found in other mammals, reptiles, and birds. The shape of the vertebral body does, howev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Orthopedic
Orthopedic surgery or orthopedics ( alternative spelling orthopaedics) is the branch of surgery concerned with conditions involving the musculoskeletal system. Orthopedic surgeons use both surgical and nonsurgical means to treat musculoskeletal trauma, spine diseases, sports injuries, degenerative diseases, infections, tumors and congenital disorders. Etymology Nicholas Andry coined the word in French as ', derived from the Ancient Greek words ("correct", "straight") and ("child"), and published ''Orthopedie'' (translated as ''Orthopædia: Or the Art of Correcting and Preventing Deformities in Children'') in 1741. The word was assimilated into English as ''orthopædics''; the ligature ''æ'' was common in that era for ''ae'' in Greek- and Latin-based words. As the name implies, the discipline was initially developed with attention to children, but the correction of spinal and bone deformities in all stages of life eventually became the cornerstone of orthopedic pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Arthroplasty
Arthroplasty (literally " e-orming of joint") is an orthopedic surgical procedure where the articular surface of a musculoskeletal joint is replaced, remodeled, or realigned by osteotomy or some other procedure. It is an elective procedure that is done to relieve pain and restore function to the joint after damage by arthritis or some other type of trauma. Types For the last 45 years, the most successful and common form of arthroplasty is the surgical replacement of arthritic or destructive or necrotic joint or joint surface with a prosthesis. For example, a hip joint that is affected by osteoarthritis may be replaced entirely ( total hip arthroplasty) with a prosthetic hip. This would involve replacing both the acetabulum (hip socket) and the head and neck of the femur. The purpose of this procedure is to relieve pain, to restore range of motion and to improve walking ability, thus leading to the improvement of muscle strength. Other types of arthroplasty * Interposit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Mitsui Chemicals
is a Japanese chemicals company listed on the Nikkei with business interests in Japan, Europe, China, Southeast Asia and the USA. It is one of the leading chemical companies in Japan and is part of the Mitsui conglomerate. The company mainly deals in performance materials, petrochemicals and basic chemicals and functional polymeric materials. History Mitsui Chemicals of the Mitsui group emerged in 1968 with the merger of Toyo Koatsu and Mitsui Chemical Industry. The former was established in 1933 and was mainly engaged in the manufacture of fertilizers while the latter was founded in 1941 and produced dyes and organic intermediates. The new entity focused in the production of four product lines: fertilizers; basic products (methanol, formaldehyde, phenol, aniline, bisphenol A, TDI, MDI, and melamine); polymers; and, fine chemicals. With the growth of opportunities in India, Mitsui Chemicals has decided to establish its first polypropylene compounding plant in India at 'Japa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |