|
Calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin ''calx'' " lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in food ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium In Biology
Calcium ions (Ca2+) contribute to the physiology and biochemistry of organisms' cells. They play an important role in signal transduction pathways, where they act as a second messenger, in neurotransmitter release from neurons, in contraction of all muscle cell types, and in fertilization. Many enzymes require calcium ions as a cofactor, including several of the coagulation factors. Extracellular calcium is also important for maintaining the potential difference across excitable cell membranes, as well as proper bone formation. Plasma calcium levels in mammals are tightly regulated, electronic-book electronic- with bone acting as the major mineral storage site. Calcium ions, Ca2+, are released from bone into the bloodstream under controlled conditions. Calcium is transported through the bloodstream as dissolved ions or bound to proteins such as serum albumin. Parathyroid hormone secreted by the parathyroid gland regulates the resorption of Ca2+ from bone, reabsorption ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Supplementation
Calcium supplements are salts of calcium used in a number of conditions. Supplementation is generally only required when there is not enough calcium in the diet. By mouth they are used to treat and prevent low blood calcium, osteoporosis, and rickets. By injection into a vein they are used for low blood calcium that is resulting in muscle spasms and for high blood potassium or magnesium toxicity. Common side effects include constipation and nausea. When taken by mouth high blood calcium is uncommon. Calcium supplements, unlike calcium from dietary sources, appear to increase the risk of kidney stones. Adults generally require about a gram of calcium a day. Calcium is particularly important for bones, muscles, and nerves. The medical use of calcium supplements began in the 19th century. It is on the World Health Organization's List of Essential Medicines. It is available as a generic medication. In 2020, it was the 204th most commonly prescribed medication in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline Earth Metal
The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are all shiny, silvery-white, somewhat reactive metals at standard temperature and pressure. Structurally, they (together with helium) have in common an outer s-orbital which is full; that is, this orbital contains its full complement of two electrons, which the alkaline earth metals readily lose to form cations with charge +2, and an oxidation state of +2. All the discovered alkaline earth metals occur in nature, although radium occurs only through the decay chain of uranium and thorium and not as a primordial element. There have been experiments, all unsuccessful, to try to synthesize element 120, the next potential member of the group. Characteristics Chemical As with other groups, the members of this family show patterns in their ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, blackboard or sidewalk chalk, and drywall. Alabaster, a fine-grained white or lightly tinted variety of gypsum, has been used for sculpture by many cultures including Ancient Egypt, Mesopotamia, Ancient Rome, the Byzantine Empire, and the Nottingham alabasters of Medieval England. Gypsum also crystallizes as translucent crystals of selenite. It forms as an evaporite mineral and as a hydration product of anhydrite. The Mohs scale of mineral hardness defines gypsum as hardness value 2 based on scratch hardness comparison. Etymology and history The word '' gypsum'' is derived from the Greek word (), "plaster". Because the quarries of the Montmartre district of Paris have long furnished burnt gypsum ( calcined gypsum) used for various purposes, this dehydrated gypsum be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neurons
A neuron, neurone, or nerve cell is an electrically excitable cell that communicates with other cells via specialized connections called synapses. The neuron is the main component of nervous tissue in all animals except sponges and placozoa. Non-animals like plants and fungi do not have nerve cells. Neurons are typically classified into three types based on their function. Sensory neurons respond to stimuli such as touch, sound, or light that affect the cells of the sensory organs, and they send signals to the spinal cord or brain. Motor neurons receive signals from the brain and spinal cord to control everything from muscle contractions to glandular output. Interneurons connect neurons to other neurons within the same region of the brain or spinal cord. When multiple neurons are connected together, they form what is called a neural circuit. A typical neuron consists of a cell body (soma), dendrites, and a single axon. The soma is a compact structure, and the axon an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
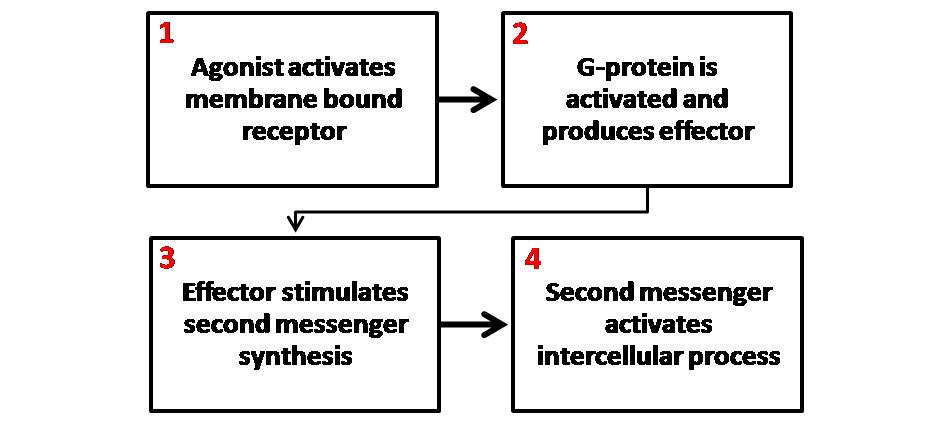
Second Messenger
Second messengers are intracellular signaling molecules released by the cell in response to exposure to extracellular signaling molecules—the first messengers. (Intercellular signals, a non-local form or cell signaling, encompassing both first messengers and second messengers, are classified as autocrine, juxtacrine, paracrine, and endocrine depending on the range of the signal.) Second messengers trigger physiological changes at cellular level such as proliferation, differentiation, migration, survival, apoptosis and depolarization. They are one of the triggers of intracellular signal transduction cascades. Examples of second messenger molecules include cyclic AMP, cyclic GMP, inositol triphosphate, diacylglycerol, and calcium. First messengers are extracellular factors, often hormones or neurotransmitters, such as epinephrine, growth hormone, and serotonin. Because peptide hormones and neurotransmitters typically are biochemically hydrophilic molecules, these first m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limestone
Limestone ( calcium carbonate ) is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of . Limestone forms when these minerals precipitate out of water containing dissolved calcium. This can take place through both biological and nonbiological processes, though biological processes, such as the accumulation of corals and shells in the sea, have likely been more important for the last 540 million years. Limestone often contains fossils which provide scientists with information on ancient environments and on the evolution of life. About 20% to 25% of sedimentary rock is carbonate rock, and most of this is limestone. The remaining carbonate rock is mostly dolomite, a closely related rock, which contains a high percentage of the mineral dolomite, . ''Magnesian limestone'' is an obsolete and poorly-defined term used variously for dolomite, for lim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apatite
Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of OH−, F− and Cl− ions, respectively, in the crystal. The formula of the admixture of the three most common endmembers is written as Ca10( PO4)6(OH,F,Cl)2, and the crystal unit cell formulae of the individual minerals are written as Ca10(PO4)6(OH)2, Ca10(PO4)6F2 and Ca10(PO4)6Cl2. The mineral was named apatite by the German geologist Abraham Gottlob Werner in 1786, although the specific mineral he had described was reclassified as fluorapatite in 1860 by the German mineralogist Karl Friedrich August Rammelsberg. Apatite is often mistaken for other minerals. This tendency is reflected in the mineral's name, which is derived from the Greek word ἀπατάω (apatáō), which means ''to deceive''. Geology Apatite is very common as an accessory mineral in igneous and metamorphic rocks, where it is the most common phosphate mineral. However, o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Signal Transduction
Signal transduction is the process by which a chemical or physical signal is transmitted through a cell as a series of molecular events, most commonly protein phosphorylation catalyzed by protein kinases, which ultimately results in a cellular response. Proteins responsible for detecting stimuli are generally termed receptors, although in some cases the term sensor is used. The changes elicited by ligand binding (or signal sensing) in a receptor give rise to a biochemical cascade, which is a chain of biochemical events known as a signaling pathway. When signaling pathways interact with one another they form networks, which allow cellular responses to be coordinated, often by combinatorial signaling events. At the molecular level, such responses include changes in the transcription or translation of genes, and post-translational and conformational changes in proteins, as well as changes in their location. These molecular events are the basic mechanisms controlling cell gro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lime (material)
Lime is a calcium-containing inorganic material composed primarily of oxides and hydroxide, usually calcium oxide and/or calcium hydroxide. It is also the name for calcium oxide which occurs as a product of coal-seam fires and in altered limestone xenoliths in volcanic ejecta. The International Mineralogical Association recognizes lime as a mineral with the chemical formula of CaO. The word ''lime'' originates with its earliest use as building mortar and has the sense of ''sticking or adhering''. These materials are still used in large quantities as building and engineering materials (including limestone products, cement, concrete, and mortar), as chemical feedstocks, and for sugar refining, among other uses. Lime industries and the use of many of the resulting products date from prehistoric times in both the Old World and the New World. Lime is used extensively for wastewater treatment with ferrous sulfate. The rocks and minerals from which these materials are derived, ty ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound or metallic ion that is required for an enzyme's role as a catalyst (a catalyst is a substance that increases the rate of a chemical reaction). Cofactors can be considered "helper molecules" that assist in biochemical transformations. The rates at which these happen are characterized in an area of study called enzyme kinetics. Cofactors typically differ from ligands in that they often derive their function by remaining bound. Cofactors can be divided into two types: inorganic ions and complex organic molecules called coenzymes. Coenzymes are mostly derived from vitamins and other organic essential nutrients in small amounts. (Note that some scientists limit the use of the term "cofactor" for inorganic substances; both types are included here.) Coenzymes are further divided into two types. The first is called a "prosthetic group", which consists of a coenzyme that is tightly (or even covalently) and permanently bound to a protein ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Human Body
The human body is the structure of a human being. It is composed of many different types of cells that together create tissues and subsequently organ systems. They ensure homeostasis and the viability of the human body. It comprises a head, hair, neck, trunk (which includes the thorax and abdomen), arms and hands, legs and feet. The study of the human body involves anatomy, physiology, histology and embryology. The body varies anatomically in known ways. Physiology focuses on the systems and organs of the human body and their functions. Many systems and mechanisms interact in order to maintain homeostasis, with safe levels of substances such as sugar and oxygen in the blood. The body is studied by health professionals, physiologists, anatomists, and by artists to assist them in their work. Composition The human body is composed of elements including hydrogen, oxygen, carbon, calcium and phosphorus. These elements reside in trillions of cells and non-cellula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |