|
Aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology. Structure and bonding Aldehyde molecules have a central carbon atom that is connected by a double bond to oxygen, a single bond to hydrogen and another single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The bond length is about 120–122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes such as formaldehyde and acetaldehyde are solubl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as aqueous solutions (formalin), which consists mainly of the hydrate CH2(OH)2. It is the simplest of the aldehydes (). As a precursor to many other materials and chemical compounds, in 2006 the global production of formaldehyde was estimated at 12 million tons per year. It is mainly used in the production of industrial resins, e.g., for particle board and coatings. Formaldehyde also occurs naturally. It is derived from the degradation of serine, dimethylglycine, and lipids. Demethylases act by converting N-methyl groups to formaldehyde. Formaldehyde is classified as a group 1 carcinogen and can cause respiratory and skin irritation upon exposure. Forms Formaldehyde is more complicated than many simple carbon compounds in that i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic compound, organic chemical compound with the chemical formula, formula , sometimes abbreviated as . It is a colorless liquid or gas, boiling near room temperature. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants. It is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol (drug), alcohol consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke. Consumption of disulfiram inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body. International Agency for Research on Cancer, The International Agency for Research on Cancer (IARC) has listed acetaldehyde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Aldehyde General Structure
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated Alcohol (chemistry), alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology. Structure and bonding Aldehyde molecules have a central carbon atom that is connected by a double bond to oxygen, a single bond to hydrogen and another single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2-Orbital hybridisation, hybridized. The aldehyde group is somewhat polar molecule, polar. The bond length is about 120–122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
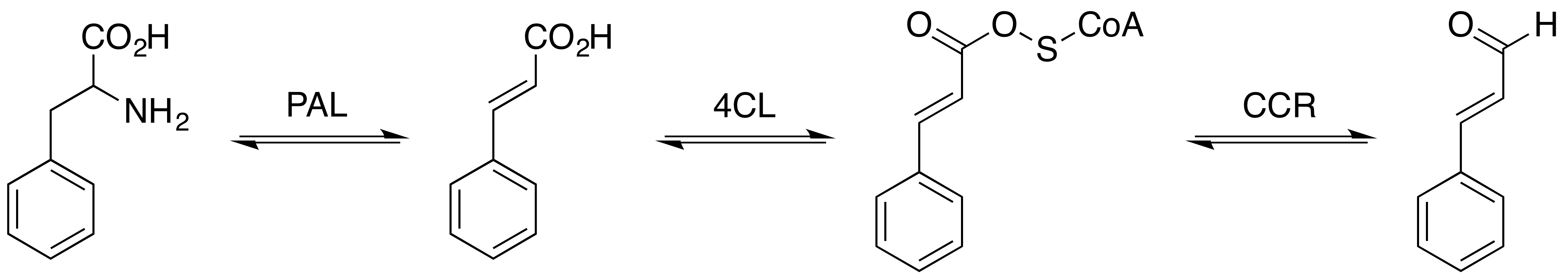
Cinnamaldehyde
Cinnamaldehyde is an organic compound with the formula or . Occurring naturally as predominantly the ''trans'' (''E'') isomer, it gives cinnamon its flavor and odor. It is a phenylpropanoid that is naturally synthesized by the shikimate pathway. This pale yellow, viscous liquid occurs in the bark of cinnamon trees and other species of the genus '' Cinnamomum''. It is an essential oil. The bark of cinnamon tree contains high concentrations of cinnamaldehyde. Structure and synthesis Cinnamaldehyde was isolated from cinnamon essential oil in 1834 by Jean-Baptiste Dumas and Eugène-Melchior Péligot and synthesized in the laboratory by the Italian chemist Luigi Chiozza in 1854. The natural product is '' trans''-cinnamaldehyde. The molecule consists of a benzene ring attached to an unsaturated aldehyde. Cinnamaldehyde is an α,β-unsaturated carbonyl compound. Its color is due to the π → π* transition: increased conjugation in comparison with acrolein shifts this band towa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hydroformylation
In organic chemistry, hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes () from alkenes (). This chemical reaction entails the net addition of a formyl group () and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention: production capacity reached 6.6 tons in 1995. It is important because aldehydes are easily converted into many secondary products. For example, the resultant aldehydes are hydrogenated to Alcohol (chemistry), alcohols that are converted to detergents. Hydroformylation is also used in speciality chemicals, relevant to the organic synthesis of fragrances and pharmaceuticals. The development of hydroformylation is one of the premier achievements of 20th-century Chemical industry, industrial chemistry. The process entails treatment of an alkene typically with high pressures (between 10 and 100 Atmosphere (unit), atmospheres) of carbon monoxi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Butyraldehyde
Butyraldehyde, also known as butanal, is an organic compound with the formula CH3(CH2)2CHO. This compound is the aldehyde derivative of butane. It is a colorless flammable liquid with an unpleasant smell. It is miscible with most organic solvents. Production Butyraldehyde is produced almost exclusively by the hydroformylation of propylene: : CH3CH=CH2 + H2 + CO → CH3CH2CH2CHO Traditionally, hydroformylation was catalyzed by cobalt carbonyl but rhodium complexes are more common. The dominant technology involves the use of rhodium catalysts derived from the water-soluble ligand tppts. An aqueous solution of the rhodium catalyst converts the propylene to the aldehyde, which forms a lighter (less dense) immiscible phase. About 6 billion kilograms are produced annually in this manner. Butyraldehyde can be produced by the catalytic dehydrogenation of N-Butanol, ''n''-butanol. At one time, it was produced industrially by the catalytic hydrogenation of crotonaldehyde, which is derived ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Vanillin
Vanillin is an organic compound with the molecular formula . It is a phenolic aldehyde. Its functional groups include aldehyde, hydroxyl, and ether. It is the primary component of the ethanolic extract of the vanilla bean. Synthetic vanillin is now used more often than natural vanilla extract as a flavoring in foods, beverages, and pharmaceuticals. Vanillin and ethylvanillin are used by the food industry; ethylvanillin is more expensive, but has a stronger note. It differs from vanillin by having an ethoxy group (−O−CH2CH3) instead of a methoxy group (−O−CH3). Natural vanilla extract is a mixture of several hundred different compounds in addition to vanillin. Artificial vanilla flavoring is often a solution of pure vanillin, usually of synthetic origin. Because of the scarcity and expense of natural vanilla extract, synthetic preparation of its predominant component has long been of interest. The first commercial synthesis of vanillin began with the more readily ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Formylation Reaction
Formylation refers to any chemical processes in which a compound is functionalized with a formyl group (-CH=O). In organic chemistry, the term is most commonly used with regards to aromatic compounds (for example the conversion of benzene to benzaldehyde in the Gattermann–Koch reaction). In biochemistry the reaction is catalysed by enzymes such as formyltransferases. Formylation generally involves the use of formylation agents, reagents that give rise to the CHO group. Among the many formylation reagents, particularly important are formic acid and carbon monoxide. A formylation reaction in organic chemistry refers to organic reactions in which an organic compound is functionalized with a formyl group (-CH=O). The reaction is a route to aldehydes (''C''-CH=O), formamides (''N''-CH=O), and formate#Formate esters, formate esters (''O''-CH=O). Formylation agents A reagent that delivers the formyl group is called a formylating agent. * Formic acid * Dimethylformamide and phosphorus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Aldose
An aldose is a monosaccharide (a simple sugar) with a carbon backbone chain with a carbonyl group on the endmost carbon atom, making it an aldehyde, and hydroxyl groups connected to all the other carbon atoms. Aldoses can be distinguished from ketoses, which have the carbonyl group away from the end of the molecule, and are therefore ketones. Structure Like most carbohydrates, simple aldoses have the general chemical formula C''n''(H2O)''n''. Because formaldehyde (n=1) and glycolaldehyde (n=2) are not generally considered to be carbohydrates, the simplest possible aldose is the triose glyceraldehyde, which only contains three carbon atoms. Because they have at least one asymmetric carbon center, all aldoses exhibit stereoisomerism. Aldoses can exist in either a - form or - form. The determination is made based on the chirality of the asymmetric carbon furthest from the aldehyde end, namely the second-last carbon in the chain. Aldoses with alcohol groups on the right of the Fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight. It is used by plants to make cellulose, the most abundant carbohydrate in the world, for use in cell walls, and by all living Organism, organisms to make adenosine triphosphate (ATP), which is used by the cell as energy. In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as amylose and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form is -glucose, while its Stereoisomerism, stereoisomer L-glucose, -glucose is produced synthetically in comparatively small amounts and is less biologicall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Vinyl Alcohol
Vinyl alcohol, also called ethenol (IUPAC name; not ethanol) or ethylenol, is the simplest enol. With the formula , it is a labile compound that converts to acetaldehyde immediately upon isolation near room temperature. It is not a practical precursor to any compound. Synthesis Vinyl alcohol can be formed by the pyrolytic elimination of water from ethylene glycol at a temperature of 900 °C and low pressure. Such processes are of no practical importance. Tautomerization of vinyl alcohol to acetaldehyde Under normal conditions, vinyl alcohol converts (tautomerizes) to acetaldehyde: : At room temperature, acetaldehyde () is more stable than vinyl alcohol () by 42.7 kJ/mol. Vinyl alcohol gas isomerizes to the aldehyde with a half-life of 30 min at room temperature. : The uncatalyzed keto–enol tautomerism by a 1,3-hydrogen migration is forbidden by the Woodward–Hoffmann rules and therefore has a high activation barrier and is not a significant pathway at or near room ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Alcohol (chemistry)
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugar alcohols and cholesterol. The presence of an OH group strongly modifies the properties of Hydrocarbon, hydrocarbons, conferring Hydrophile, hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur. History The flammable nature of the exhalations of wine was already known to ancient natural philosophers such as Aristotle (384–322 BCE), Theophrastus (–287 BCE), and Pliny the Elder (23/24–79 CE). However, this did not immediately lead to the isolation of alcohol, even despite the development of more advanced distillation techniques in second- and third-century Roman Egypt. An important recognition, first found in one of the writings attributed to Jabir ibn Hayyan, J� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |