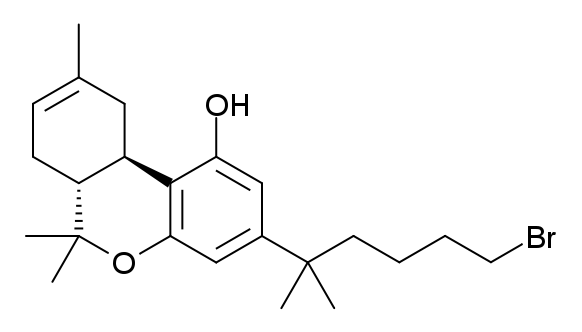
|
AM-2201
AM-2201 (1-(5-fluoropentyl)-3-(1-naphthoyl)indole) is a recreational designer drug that acts as a potent but nonselective full agonist for the cannabinoid receptor. It is part of the AM series of cannabinoids discovered by Alexandros Makriyannis at Northeastern University. Hazards Convulsions have been reported including at doses as low as 10 mg. Pharmacology AM-2201 is a full agonist for cannabinoid receptors. Affinities are: with a ''K''i of 1.0 nM at CB1 and 2.6 nM at CB2. The 4-methyl functional analog MAM-2201 probably has similar affinities. AM-2201 has an EC50 of 38 nM for human CB1 receptors, and 58 nM for human CB2 receptors. AM-2201 produces bradycardia and hypothermia in rats at doses of 0.3–3 mg/kg, comparable to the potency of JWH-018 in rats, suggesting potent cannabinoid-like activity. Pharmacokinetics AM-2201 metabolism differs only slightly from that of JWH-018. AM-2201 ''N''-dealkylation produces fluoropentane instead of p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MAM-2201
MAM-2201 (4'-methyl-AM-2201, 5"-fluoro-JWH-122) is a drug that presumably acts as a potent agonist for the cannabinoid receptors. It had never previously been reported in the scientific or patent literature, and was first identified by laboratories in the Netherlands and Germany in June 2011 as an ingredient in synthetic cannabis smoking blends. Like RCS-4 and AB-001, MAM-2201 thus appears to be a novel compound invented by "research chemical" suppliers specifically for grey-market recreational use. Structurally, MAM-2201 is a hybrid of two known cannabinoid compounds JWH-122 and AM-2201, both of which had previously been used as active ingredients in synthetic cannabis blends before being banned in many countries. A study of MAM-2201 in rats showed that it causes neurofunctional disruptions. Legal status In the United States, all CB1 receptor agonists of the 3-(1-naphthoyl)indole class such as MAM-2201 are Schedule I Controlled Substances. MAM-2201 has been banned by being add ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
JWH-018
JWH-018 (1-pentyl-3-(1-naphthoyl)indole, NA-PIMO or AM-678) is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 cannabinoid receptors, with some selectivity for CB2. It produces effects in animals similar to those of tetrahydrocannabinol (THC), a cannabinoid naturally present in cannabis, leading to its use in synthetic cannabis products that in some countries are sold legally as "incense blends". As a full agonist at both the CB1 and CB2 cannabinoid receptors, this chemical compound is classified as an analgesic medication. The analgesic effects of cannabinoid ligands, mediated by CB1 receptors are well established in treatment of neuropathic pain, as well as cancer pain and arthritis. These compounds work by mimicking the body's naturally-produced endocannabinoid hormones such as 2-AG and anandamide (AEA), which are biologically active and can exacerbate or inhibit nerve signaling. As the cause is poorly understood in chr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM Cannabinoids
Alexandros Makriyannis is a professor in the Department of Medicinal Chemistry at Northeastern University, where his research group has synthesized many new compounds with cannabinoid activity. Some of those are: See also * List of CP cannabinoids * List of JWH cannabinoids * List of HU cannabinoids * List of miscellaneous designer cannabinoids Since the first synthetic cannabinoids were discovered in recreational drug products in 2008, new synthetic cannabinoids with no precedent in the scientific literature continue to be identified. These synthetic cannabinoids appear to be rationally ... References {{Cannabinoids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NM-2201
NM-2201 (also known as CBL-2201) is an indole-based synthetic cannabinoid that presumably has similar properties to the closely related 5F-PB-22 and NNE1, which are both full agonists and unselectively bind to CB1 and CB2 receptors with low nanomolar affinity. Pharmacology NM-2201 acts as a full agonist with a binding affinity of 0.332 nM at CB1 and 0.732 nM at CB2 cannabinoid receptors. It has been linked to serious adverse events in users. Legal status NM-2201 is specifically banned in Sweden, Germany (Anlage II), and Japan but is also controlled in many other jurisdictions under analogue laws. On May 30, 2018 the United States Drug Enforcement Administration, Department of Justice published a notice of intent to place NM-2201 and 4 other synthetic cannabinoids in schedule I of the Controlled Substances Act. This notice went into effect on June 29, 2018. Use NM-2201 was linked to an incident in December 2015 where 25-30 people in Ocala, FL were taken to hos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of AM Cannabinoids
Alexandros Makriyannis is a professor in the Department of Medicinal Chemistry at Northeastern University, where his research group has synthesized many new compounds with cannabinoid activity. Some of those are: See also * List of CP cannabinoids * List of JWH cannabinoids * List of HU cannabinoids * List of miscellaneous designer cannabinoids Since the first synthetic cannabinoids were discovered in recreational drug products in 2008, new synthetic cannabinoids with no precedent in the scientific literature continue to be identified. These synthetic cannabinoids appear to be rationally ... References {{Cannabinoids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
THJ-2201
THJ-2201 is an indazole-based synthetic cannabinoid that presumably acts as a potent agonist of the CB1 receptor and has been sold online as a designer drug. It is a structural analog of AM-2201 in which the central indole ring has been replaced by indazole. Pharmacology THJ-2201 acts as a full agonist with a binding affinity of 1.34nM at CB1 and 1.32nM at CB2 cannabinoid receptors. Side effects THJ-2201 has been linked to at least one hospitalization and death due to its use. Legal status Because of the hazards associated with recreational use of this compound, it is classified as a Schedule I controlled substance in the United States. It is also an Anlage II controlled drug in Germany. See also * AM-694 * AM-1235 * AM-2232 * AM-2233 * FUBIMINA * JWH-018 * List of AM cannabinoids * List of JWH cannabinoids * NM-2201 * THJ-018 THJ-018 (SGT-17) is a synthetic cannabinoid that is the indazole analogue of JWH-018 and has been sold online as a designer drug. Pharmaco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
THJ-018
THJ-018 (SGT-17) is a synthetic cannabinoid that is the indazole analogue of JWH-018 and has been sold online as a designer drug. Pharmacology THJ-018 acts as a full agonist with a binding affinity of 5.84 nM at CB1 and 4.57 nM at CB2 cannabinoid receptors. Legality THJ-018 is an Anlage II controlled drug in Germany. It is also banned in Sweden. See also * THJ-2201 * AM-2201 AM-2201 (1-(5-fluoropentyl)-3-(1-naphthoyl)indole) is a recreational designer drug that acts as a potent but nonselective full agonist for the cannabinoid receptor. It is part of the AM series of cannabinoids discovered by Alexandros Makriyannis ... References Cannabinoids Designer drugs Naphthoylindazoles {{cannabinoid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SDB-005
SDB-005 is an indazole-based synthetic cannabinoid that has been sold online as a designer drug. It is presumed to be an agonist of the CB1 and CB2 cannabinoid receptors. SDB-005 is the indazole core analog of PB-22 where the 8-hydroxyquinoline has also been replaced with a naphthalene group. The code number SDB-005 was originally used for a different compound, the ''N''-phenyl instead of ''N''-benzyl analogue of SDB-006. This compound is a potent agonist of the CB1 receptor (Ki = 21 nM) and CB2 receptor (Ki = 140 nM). However, SDB-005 was subsequently used as the name for the indazole-3-carboxylate compound mentioned above when it was sold in Europe as a designer drug, and was entered into the EMCDDA synthetic drug database under this name. Consequently, there are now two distinct, yet fairly closely related cannabinoid compounds, which may both be referred to under the code SDB-005. See also * 5F-PB-22 * AM-2201 * BB-22 * JWH-018 * NM-2201 * NNE1 NNE1 (also known as N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-1235
AM-1235 (1-(5-fluoropentyl)-3-(naphthalen-1-oyl)-6-nitroindole) is a drug that acts as a potent and reasonably selective agonist for the cannabinoid receptor CB1. Pharmacology Pharmacodynamics AM-1235 is a cannabinoid receptor agonist with Ki of 1.5 nM at CB1 compared to 20.4 nM at CB2. While the 6-nitro substitution on the indole ring reduces affinity for both CB1 and CB2 relative to the unsubstituted parent compound AM-2201, CB2 affinity is reduced much more, resulting in a CB1 selectivity of around 13 times. This is in contrast to other related compounds such as AM-1221 where a 6-nitro substitution instead confers significant selectivity for CB2. Pharmacokinetics AM-1235 metabolism differs only slightly from that of JWH-018. AM-1235 ''N''-dealkylation produces fluoropentane instead of pentane (or plain alkanes in general). It has been speculated that the fluoropentane might function as an alkylating agent or is further metabolized into toxic fluoroacetic acid. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-694
AM-694 (1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole) is a designer drug that acts as a potent and selective agonist for the cannabinoid receptor CB1. It is used in scientific research for mapping the distribution of CB1 receptors. Pharmacology AM-694 is an agonist for cannabinoid receptors. It has a ''K''i of 0.08 nM at CB1 and 18 times selectivity over CB2 with a ''K''i of 1.44 nM. It is unclear what is responsible for this unusually high CB1 binding affinity, but it makes the 18F radiolabelled derivative of AM-694 useful for mapping the distribution of CB1 receptors in the body. Metabolism Pathways of metabolism include hydrolytic defluorination, carboxylation, and monohydroxylation of the ''N''-alkyl chain. See also * AM-679 * AM-1235 * AM-2201 * AM-2232 * AM-2233 * FUBIMINA * JWH-018 * List of AM cannabinoids * List of JWH cannabinoids * THJ-2201 THJ-2201 is an indazole-based synthetic cannabinoid that presumably acts as a potent agonist of the CB1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Schedule I Drugs (US)
This is the list of Schedule I drugs as defined by the United States Controlled Substances Act. 21 CFRbr>1308.11(CSA Sched I) with changes through (Oct 18, 2012). Retrieved September 6, 2013. The following findings are required for drugs to be placed in this schedule: # The drug or other substance has a high potential for abuse. # The drug or other substance has no currently accepted medical use in treatment in the United States. # There is a lack of accepted safety for use of the drug or other substance under medical supervision. Except as specifically authorized, it is illegal for any person: # to manufacture, distribute, or dispense, or possess with intent to manufacture, distribute, or dispense, a controlled substance; or # to create, distribute, dispense, or possess with intent to distribute or dispense, a counterfeit substance. Additional substances are added to the list by the Secretary of Health and Human Services pursuant to 21 CFR 1308.49. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Recreational Drug
Recreational drug use indicates the use of one or more psychoactive drugs to induce an altered state of consciousness either for pleasure or for some other casual purpose or pastime by modifying the perceptions and emotions of the user. When a psychoactive drug enters the user's body, it induces an intoxicating effect. Generally, recreational drugs are divided into three categories: depressants (drugs that induce a feeling of relaxation and calmness); stimulants (drugs that induce a sense of energy and alertness); and hallucinogens (drugs that induce perceptual distortions such as hallucination). In popular practice, recreational drug use generally is a tolerated social behaviour, rather than perceived as the medical condition of self-medication. However, heavy use of some drugs is socially stigmatized. Many people also use prescribed and controlled depressants such as opioids, as well as opiates and benzodiazepines. Common recreational drugs include caffeine, commonly foun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |