three-center two-electron bond on:
[Wikipedia]
[Google]
[Amazon]
A three-center two-electron (3c–2e) bond is an electron-deficient
 The monomer BH3 is unstable since the boron atom has an empty p-orbital. A B−H−B 3-center-2-electron bond is formed when a boron atom shares electrons with a B−H bond on another boron atom. The two electrons (corresponding to one bond) in a B−H−B bonding molecular orbital are spread out across three internuclear spaces.
In
The monomer BH3 is unstable since the boron atom has an empty p-orbital. A B−H−B 3-center-2-electron bond is formed when a boron atom shares electrons with a B−H bond on another boron atom. The two electrons (corresponding to one bond) in a B−H−B bonding molecular orbital are spread out across three internuclear spaces.
In 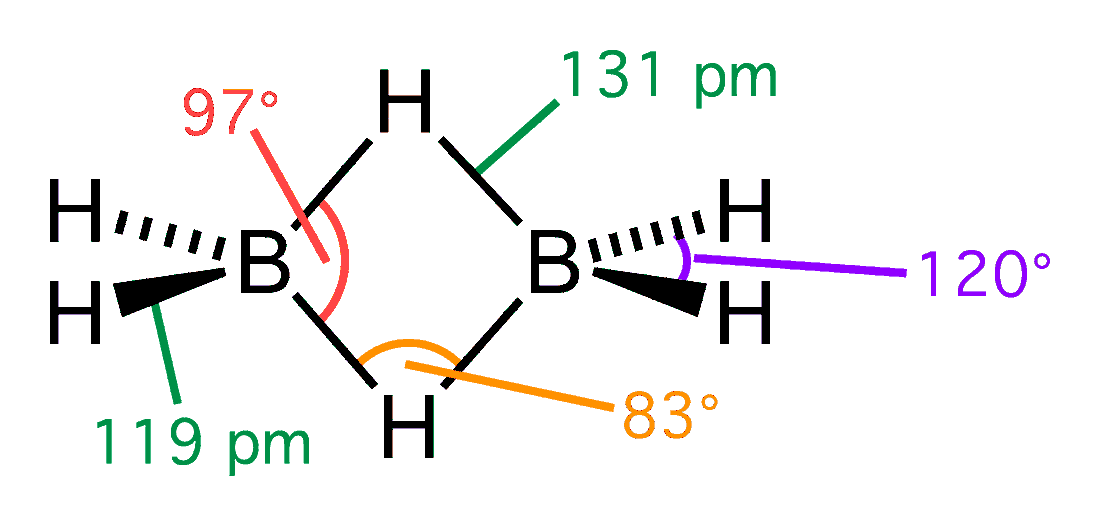
 Three-center, two-electron bonding is pervasive in organotransition metal chemistry. A celebrated family of compounds featuring such interactions as called
Three-center, two-electron bonding is pervasive in organotransition metal chemistry. A celebrated family of compounds featuring such interactions as called
chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
where three atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s share two electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
s. The combination of three atomic orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any spe ...
s form three molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of findin ...
s: one bonding, one ''non''-bonding, and one ''anti''-bonding. The two electrons go into the bonding orbital, resulting in a net bonding effect and constituting a chemical bond among all three atoms. In many common bonds of this type, the bonding orbital is shifted towards two of the three atoms instead of being spread equally among all three. Example molecules with 3c–2e bonds are the trihydrogen cation
The trihydrogen cation or protonated molecular hydrogen is a cation (positive ion) with formula , consisting of three hydrogen nuclei (protons) sharing two electrons.
The trihydrogen cation is one of the most abundant ions in the universe. It is ...
() and diborane
Diborane(6), generally known as diborane, is the chemical compound with the formula B2H6. It is a toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Diborane is a key boron compound with a variety of applications. It has attracte ...
(). In these two structures, the three atoms in each 3c-2e bond form an angular geometry, leading to a bent bond
In organic chemistry, a bent bond, also known as a banana bond, is a type of covalent chemical bond with a geometry somewhat reminiscent of a banana. The term itself is a general representation of electron density or configuration resembling a ...
.
Boranes and carboranes
An extended version of the 3c–2e bond model features heavily incluster compound
In chemistry, an atom cluster (or simply cluster) is an ensemble of bound atoms or molecules that is intermediate in size between a simple molecule and a nanoparticle; that is, up to a few nanometers (nm) in diameter. The term ''microcluster' ...
s described by the polyhedral skeletal electron pair theory, such as boranes
Boranes is the name given to compounds with the formula BxHy and related anions. Many such boranes are known. Most common are those with 1 to 12 boron atoms. Although they have few practical applications, the boranes exhibit structures and bond ...
and carborane
Carboranes are electron-delocalized (non-classically bonded) clusters composed of boron, carbon and hydrogen atoms.Grimes, R. N., ''Carboranes 3rd Ed.'', Elsevier, Amsterdam and New York (2016), . Like many of the related boron hydrides, these c ...
s. These molecules derive their stability from having a completely filled set of bonding molecular orbitals as outlined by Wade's rules.
diborane
Diborane(6), generally known as diborane, is the chemical compound with the formula B2H6. It is a toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Diborane is a key boron compound with a variety of applications. It has attracte ...
(B2H6), there are two such 3c-2e bonds: two H atoms bridge the two B atoms, leaving two additional H atoms in ordinary B−H bonds on each B. As a result, the molecule achieves stability since each B participates in a total of four bonds and all bonding molecular orbitals are filled, although two of the four bonds are 3-center B−H−B bonds. The reported bond order for each B−H interaction in a bridge is 0.5,F. Albert Cotton
Frank Albert Cotton FRS (April 9, 1930 – February 20, 2007) was an American chemist. He was the W.T. Doherty-Welch Foundation Chair and Distinguished Professor of Chemistry at Texas A&M University. He authored over 1600 scientific articles. C ...
, Geoffrey Wilkinson
Sir Geoffrey Wilkinson FRS (14 July 1921 – 26 September 1996) was a Nobel laureate English chemist who pioneered inorganic chemistry and homogeneous transition metal catalysis.
Education and early life
Wilkinson was born at Springside, Todm ...
and Paul L. Gaus, ''Basic Inorganic Chemistry'', 2nd ed. (Wiley 1987), p.113 so that the bridging B−H−B bonds are weaker and longer than the terminal B−H bonds, as shown by the bond length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of ...
s in the structural diagram.
:
Transition metal complexes
 Three-center, two-electron bonding is pervasive in organotransition metal chemistry. A celebrated family of compounds featuring such interactions as called
Three-center, two-electron bonding is pervasive in organotransition metal chemistry. A celebrated family of compounds featuring such interactions as called agostic complex
In organometallic chemistry, agostic interaction refers to the interaction of a coordinatively-unsaturated transition metal with a C−H bond, when the two electrons involved in the C−H bond enter the empty d-orbital of the transition metal, r ...
es.
Other compounds
This bonding pattern is also seen intrimethylaluminium
Trimethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2( CH3)6 (abbreviated as Al2Me6 or TMA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industriall ...
, which forms a dimer Al2(CH3)6 with the carbon atoms of two of the methyl group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in ma ...
s in bridging positions. This type of bond also occurs in carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
compounds, where it is sometimes referred to as hyperconjugation
In organic chemistry, hyperconjugation (σ-conjugation or no-bond resonance) refers to the delocalization of electrons with the participation of bonds of primarily σ-character. Usually, hyperconjugation involves the interaction of the electron ...
; another name for asymmetrical three-center two-electron bonds.
Beryllium
The first stable subvalent Be complex ever observed contains a three-center two-electron π-bond that consists of donor-acceptor interactions over the C-Be-C core of a Be(0)-carbene adduct.Carbocations
Carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountere ...
rearrangement reactions occur through three-center bond transition states. Because the three center bond structures have about the same energy as carbocations, there is generally virtually no activation energy for these rearrangements so they occur with extraordinarily high rates.
Carbonium ion
In chemistry, a carbonium ion is any cation that has a pentavalent carbon atom. The name carbonium may also be used for the simplest member of the class, properly called methanium (), where the five valences are filled with hydrogen atoms.
The ...
s such as ethanium
In chemistry, ethanium or protonated ethane is a highly reactive positive ion with formula . It can be described as a molecule of ethane () with one extra proton (hydrogen nucleus), that gives it a +1 electric charge.
Ethanium is one of the s ...
have three-center two-electron bonds. Perhaps the best known and studied structure of this sort is the 2-Norbornyl cation.
See also
*Three-center four-electron bond
The 3-center 4-electron (3c–4e) bond is a model used to explain bonding in certain hypervalent molecules such as tetratomic and hexatomic interhalogen compounds, sulfur tetrafluoride, the xenon fluorides, and the bifluoride ion. It is also know ...
* 2-Norbornyl cation
* Dihydrogen complex
Dihydrogen complexes are coordination complexes containing intact H2 as a ligand. They are a subset of sigma complexes. The prototypical complex is W(CO)3( PCy3)2(H2). This class of compounds represent intermediates in metal-catalyzed reactions ...
References
{{Chemical bonds Chemical bonding