silicate on:
[Wikipedia]
[Google]
[Amazon]
 A silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used for any
A silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used for any

 With two shared oxides bound to each silicon, cyclic or polymeric structures can result. The cyclic metasilicate ring is a hexamer of SiO32-.
With two shared oxides bound to each silicon, cyclic or polymeric structures can result. The cyclic metasilicate ring is a hexamer of SiO32-. 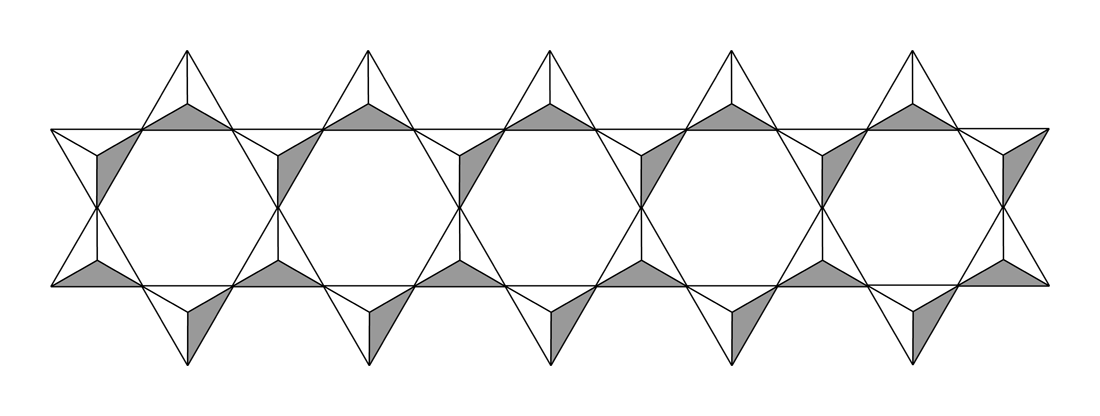 Double-chain silicates, the other category of inosilicates, occur when tetrahedra form a double chain (not always but mostly) by sharing two or three oxygen atoms each. Common minerals for this group are amphiboles.
Double-chain silicates, the other category of inosilicates, occur when tetrahedra form a double chain (not always but mostly) by sharing two or three oxygen atoms each. Common minerals for this group are amphiboles.
 In this group, known as
In this group, known as
 A silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used for any
A silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used for any salt
In common usage, salt is a mineral composed primarily of sodium chloride (NaCl). When used in food, especially in granulated form, it is more formally called table salt. In the form of a natural crystalline mineral, salt is also known as r ...
of such anions, such as sodium metasilicate; or any ester containing the corresponding chemical group, such as tetramethyl orthosilicate. The name "silicate" is sometimes extended to any anions containing silicon, even if they do not fit the general formula or contain other atoms besides oxygen; such as hexafluorosilicate . Most commonly, silicates are encountered as silicate minerals.
For diverse manufacturing, technological, and artistic needs, silicates are versatile materials, both natural (such as granite
Granite ( ) is a coarse-grained (phanerite, phaneritic) intrusive rock, intrusive igneous rock composed mostly of quartz, alkali feldspar, and plagioclase. It forms from magma with a high content of silica and alkali metal oxides that slowly coo ...
, gravel, and garnet
Garnets () are a group of silicate minerals that have been used since the Bronze Age as gemstones and abrasives.
Garnet minerals, while sharing similar physical and crystallographic properties, exhibit a wide range of chemical compositions, de ...
) and artificial (such as Portland cement, ceramics, glass
Glass is an amorphous (non-crystalline solid, non-crystalline) solid. Because it is often transparency and translucency, transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window pane ...
, and waterglass).
Structural principles
In most silicates, a silicon atom occupies the center of an idealizedtetrahedron
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...
whose corners are four oxygen atoms, connected to it by single covalent bonds according to the octet rule. The oxygen atoms, which bears some negative charge, link to other cations (Mn+). This Si-O-M-O-Si linkage is strong and rigid, which properties are manifested in the rock-like silicates. The silicates can be classified according to the length and crosslinking of the silicate anions.
Isolated silicates
Isolated orthosilicate anions have the formula . A common mineral in this group isolivine
The mineral olivine () is a magnesium iron Silicate minerals, silicate with the chemical formula . It is a type of Nesosilicates, nesosilicate or orthosilicate. The primary component of the Earth's upper mantle (Earth), upper mantle, it is a com ...
().
Two or more silicon atoms can share oxygen atoms in various ways, to form more complex anions, such as pyrosilicate .
Chains

 With two shared oxides bound to each silicon, cyclic or polymeric structures can result. The cyclic metasilicate ring is a hexamer of SiO32-.
With two shared oxides bound to each silicon, cyclic or polymeric structures can result. The cyclic metasilicate ring is a hexamer of SiO32-. Polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
ic silicate anions of can exist also as long chains.
In single-chain silicates, which are a type of inosilicate
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust.
In mineralogy, the crystalline forms of silica (silicon dio ...
, tetrahedra link to form a chain by sharing two oxygen atoms each. A common mineral in this group is pyroxene.
 Double-chain silicates, the other category of inosilicates, occur when tetrahedra form a double chain (not always but mostly) by sharing two or three oxygen atoms each. Common minerals for this group are amphiboles.
Double-chain silicates, the other category of inosilicates, occur when tetrahedra form a double chain (not always but mostly) by sharing two or three oxygen atoms each. Common minerals for this group are amphiboles.
Sheets
 In this group, known as
In this group, known as phyllosilicates
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust.
In mineralogy, the crystalline forms of silica (silicon dio ...
, tetrahedra all share three oxygen atoms each and in turn link to form two-dimensional sheets. This structure does lead to minerals in this group having one strong cleavage plane. Micas fall into this group. Both muscovite and biotite have very weak layers that can be peeled off in sheets.
Framework
In a framework silicate, known as a tectosilicate, each tetrahedron shares all 4 oxygen atoms with its neighbours, forming a 3D structure.Quartz
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The Atom, atoms are linked in a continuous framework of SiO4 silicon–oxygen Tetrahedral molecular geometry, tetrahedra, with each oxygen being shared between two tet ...
and feldspar
Feldspar ( ; sometimes spelled felspar) is a group of rock-forming aluminium tectosilicate minerals, also containing other cations such as sodium, calcium, potassium, or barium. The most common members of the feldspar group are the ''plagiocl ...
s are in this group.
Silicates with non-tetrahedral silicon
Although the tetrahedron is a common coordination geometry for silicon(IV) compounds, silicon may also occur with higher coordination numbers. For example, in the anion hexafluorosilicate , the silicon atom is surrounded by six fluorine atoms in an octahedral arrangement. This structure is also seen in the hexahydroxysilicate anion that occurs in thaumasite, a mineral found rarely in nature but sometimes observed among other calcium silicate hydrates artificially formed in cement and concrete structures submitted to a severe sulfate attack in argillaceous grounds containing oxidized pyrite. At very high pressure, such as exists in the majority of the Earth's rock, even SiO2 adopts the six-coordinated octahedral geometry in the mineral stishovite, a dense polymorph ofsilica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
found in the lower mantle of the Earth and also formed by shock during meteorite impacts.
Chemical properties
Silicates with alkali cations and small or chain-like anions, such as sodium ortho- and metasilicate, are fairly soluble in water. They form several solid hydrates when crystallized from solution. Soluble sodium silicates and mixtures thereof, known as waterglass are important industrial and household chemicals. Silicates of non-alkali cations, or with sheet and tridimensional polymeric anions, generally have negligible solubility in water at normal conditions.Reactions
Silicates are generally inert chemically. Hence they are common minerals. Their resiliency also recommends their use as building materials. When treated with calcium oxides and water, silicate minerals form Portland cement. Equilibria involving hydrolysis of silicate minerals are difficult to study. The chief challenge is the very low solubility of SiO44- and its various protonated forms. Such equilibria are relevant to the processes occurring on geological time scales.G. B. Alexander (1953): "The Reaction of Low Molecular Weight Silicic Acids with Molybdic Acid". ''Journal of the American Chemical Society, volume 75, issue 22, pages 5655–5657. Some plants excrete ligands that dissolve silicates, a step in biomineralization. Catechols can depolymerize SiO₂—a component of silicates with ionic structures like orthosilicate (SiO₄⁴⁻), metasilicate (SiO₂³⁻), and pyrosilicate (Si₂O₆⁷⁻)—by forming bis- and tris(catecholate)silicate dianions through coordination. This complexes can be further coated on various substrates for applications such as drug delivery systems, antibacterial and antifouling applications.Detection
Silicate anions in solution react with molybdate anions yielding yellow silicomolybdate complexes. In a typical preparation, monomeric orthosilicate was found to react completely in 75 seconds; dimeric pyrosilicate in 10 minutes; and higher oligomers in considerably longer time. In particular, the reaction is not observed with suspensions of colloidal silica.Zeolite formation and geopolymers polymerisation
The nature of soluble silicates is relevant to understanding biomineralization and the synthesis of aluminosilicates, such as the industrially important catalysts called zeolites. Along with aluminate anions, soluble silicate anions also play a major role in the polymerization mechanism of geopolymers. Geopolymers areamorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid) is a solid that lacks the long-range order that is a characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymousl ...
aluminosilicates whose production requires less energy than that of ordinary Portland cement. So, geopolymer cements could contribute to limiting the emissions in the Earth atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
and the global warming
Present-day climate change includes both global warming—the ongoing increase in global average temperature—and its wider effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes ...
caused by this greenhouse gas.
See also
* * Alkali-silica reaction * Carbon cycle * Carbonate-silicate cycle * Ocean acidificationReferences
{{DEFAULTSORT:Silicon-oxygen tetrahedron Silicates Silicon oxyanions