|
Aluminate
In chemistry, an aluminate is a compound containing an oxyanion of aluminium, such as sodium aluminate. In the naming of inorganic compounds, it is a suffix that indicates a polyatomic ion, polyatomic anion with a central aluminum atom. Aluminate oxyanions Aluminium oxide (alumina) is amphoteric: it dissolves in both bases and acids. When dissolved in bases it forms hydroxyaluminate ions in the same way as aluminium hydroxide or aluminium salts. The hydroxyaluminate or hydrated aluminate can be precipitated and then calcination, calcined to produce anhydrous aluminates. Aluminates are often formulated as a combination of basic oxide and aluminium oxide, for example the formula of anhydrous sodium aluminate NaAlO2 would be shown as Na2O·Al2O3. A number of aluminate oxyanions are known: * The simplest is the approximately tetrahedral found in the compound Na5AlO4, * framework ions in anhydrous sodium aluminate NaAlO2 and monocalcium aluminate, CaAl2O4 made up of corner-sharing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tricalcium Aluminate
Tricalcium aluminate Ca3Al2O6, often formulated as 3CaO·Al2O3 to highlight the proportions of the oxides from which it is made, is the most basic of the calcium aluminates. It does not occur in nature, but is an important mineral phase in Portland cement. Properties Pure tricalcium aluminate is formed when the appropriate proportions of finely divided calcium oxide and aluminium oxide are heated together above 1300 °C. The pure form is cubic, with unit cell dimension 1.5263 nm and has density 3064 kg·m−3. It melts with decomposition at 1542 °C. The unit cell contains 8 cyclic Al6O1818− anions, which can be considered to consist of 6 corner sharing AlO4 tetrahedra. The structure of pure liquid tricalcium aluminate contains mostly AlO4 tetrahedra in an infinite network, with a slightly higher concentration of bridging oxygens than expected from the composition and around 10% unconnected AlO4 monomers and Al2O7 dimers. In Portland cement clinker (cement ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Aluminates
Calcium aluminates are a range of materials obtained by heating calcium oxide and aluminium oxide together at high temperatures. They are encountered in the manufacture of refractories and cements. The stable phases shown in the phase diagram (formed at atmospheric pressure under an atmosphere of normal humidity) are: * Tricalcium aluminate, 3CaO·Al2O3 (C3A) * Dodecacalcium hepta-aluminate, 12CaO·7Al2O3 (C12A7) (once known as mayenite) * Monocalcium aluminate, CaO·Al2O3 (CA) (occurring in nature as krotite and dmitryivanovite - two polymorphs) * Monocalcium dialuminate, CaO·2Al2O3 (CA2) (occurring in nature as grossite ) * Monocalcium hexa-aluminate, CaO·6Al2O3 (CA6) (occurring in nature as hibonite, a representative of magnetoplumbite group) In addition, other phases include: * Dicalcium aluminate, 2CaO·Al2O3 (C2A), which exists only at pressures above 2500 MPa. The crystal is orthorhombic, with density 3480 kg·m−3. The natural dicalcium aluminate, b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Aluminate
Sodium aluminate is an inorganic chemical that is used as an effective source of aluminium hydroxide for many industrial and technical applications. Pure sodium aluminate (anhydrous) is a white crystalline solid having a formula variously given as NaAlO2, NaAl(OH)4 (hydrated), Na2O·Al2O3, or Na2Al2O4. Commercial sodium aluminate is available as a solution or a solid. Other related compounds, sometimes called sodium aluminate, prepared by reaction of Na2O and Al2O3 are Na5AlO4 which contains discrete AlO45− anions, Na7Al3O8 and Na17Al5O16 which contain complex polymeric anions, and NaAl11O17, once mistakenly believed to be β-alumina, a phase of aluminium oxide.Egon Wiberg, Arnold Frederick Holleman (2001) ''Inorganic Chemistry'', Elsevier Structure Anhydrous sodium aluminate, NaAlO2, contains a three-dimensional framework of corner linked AlO4 tetrahedra. The hydrated form NaAlO2·5/4H2O has layers of AlO4 tetrahedra joined into rings and the layers are held together by sodi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Aluminate
Sodium aluminate is an inorganic chemical that is used as an effective source of aluminium hydroxide for many industrial and technical applications. Pure sodium aluminate (anhydrous) is a white crystalline solid having a formula variously given as NaAlO2, NaAl(OH)4 (hydrated), Na2O·Al2O3, or Na2Al2O4. Commercial sodium aluminate is available as a solution or a solid. Other related compounds, sometimes called sodium aluminate, prepared by reaction of Na2O and Al2O3 are Na5AlO4 which contains discrete AlO45− anions, Na7Al3O8 and Na17Al5O16 which contain complex polymeric anions, and NaAl11O17, once mistakenly believed to be β-alumina, a phase of aluminium oxide.Egon Wiberg, Arnold Frederick Holleman (2001) ''Inorganic Chemistry'', Elsevier Structure Anhydrous sodium aluminate, NaAlO2, contains a three-dimensional framework of corner linked AlO4 tetrahedra. The hydrated form NaAlO2·5/4H2O has layers of AlO4 tetrahedra joined into rings and the layers are held together by sodi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Tetrachloroaluminate
Lithium tetrachloroaluminate (LAC, lithium aluminium chloride) is an inorganic compound, a tetrachloroaluminate of lithium, with the formula LiAlCl4. Solution of lithium tetrachloroaluminate in thionyl chloride is the liquid cathode and electrolyte of some lithium batteries, e.g. the lithium-thionyl chloride cell. Another cathode-electrolyte formulation is lithium tetrachloroaluminate+thionyl chloride+sulfur dioxide+bromine. Other salts used in lithium battery electrolytes are lithium bromide, lithium perchlorate, lithium tetrafluoroborate, and lithium hexafluorophosphate; less common ones are lithium chloride, lithium iodide, lithium chlorate, lithium nitrate, lithium hexafluoroarsenate, lithium hexafluorosilicate (i.e. lithium and hexafluorosilicic acid), lithium bis(trifluoromethanesulfonyl)imide Lithium bis(trifluoromethanesulfonyl)imide, often simply referred to as LiTFSI, is a hydrophilic salt with the chemical formula LiC2F6NO4S2. It is commonly used as Li-ion sourc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monocalcium Aluminate
Monocalcium aluminate (CaAl2O4) is one of the series of calcium aluminates. It does occur in nature, although only very rarely, as two polymorphs known as krotite and dmitryivanovite, both from meteorites. It is important in the composition of calcium aluminate cements. Properties Monocalcium aluminate is formed when the appropriate proportions of calcium carbonate and aluminium oxide are heated together until the mixture melts. It melts incongruently at 1390 °C. The crystal is monoclinic and pseudohexagonal, and has density 2945 kg.m−3. In calcium aluminate cements, it exists as a solid solution in which the amount of minor elements depends upon the bulk composition of the cement A cement is a binder, a chemical substance used for construction that sets, hardens, and adheres to other materials to bind them together. Cement is seldom used on its own, but rather to bind sand and gravel ( aggregate) together. Cement mi .... A typical compositionP. C. Hew ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, non-magnetic and ductile. It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe. The radioactivity of 26Al is used in radiodating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ is small and highly charged; as such, it is polarizing, and bonds aluminium forms tend towards covalency. The strong affinity tow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hexafluoroaluminate
Sodium aluminium hexafluoride is an inorganic compound with formula Na3 Al F6. This white solid, discovered in 1799 by Peder Christian Abildgaard (1740–1801), occurs naturally as the mineral cryolite and is used extensively in the industrial production of aluminium metal. The compound is the sodium (Na+) salt of the hexafluoroaluminate (AlF63−) ion. Production Most cryolite is manufactured by a variety of related pathways. One route entails combining sodium aluminate and hydrofluoric acid: :Na3Al(OH)6 + 6 HF → Na3AlF6 + 6 H2O Other routes include: : : Often the hexafluorosilicic acid, which is recovered from phosphate mining, is the precursor in a two-step process beginning with neutralization with ammonia to give ammonium hexafluorosilicate: :H3AlF6 + 3NH3 → (NH4)3AlF6 :(NH4)3AlF6 + 3NaOH → Na3AlF6 + 3NH3 + 3H2O The mineral form of sodium hexafluoroaluminate, which is called cryolite, was mined at Ivigtût on the west coast of Greenland until ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Aluminium Hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li Al H4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage. Properties, structure, preparation LAH is a colourless solid but commercial samples are usually gray due to contamination. This material can be purified by recrystallization from diethyl ether. Large-scale purifications employ a Soxhlet extractor. Commonly, the impure gray material is used in synthesis, since the impurities are innocuous and can be easily separated from the organic products. The pure powdered material is pyrophoric, but not its large crystals. Some commercial materials contain mineral oil to in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Hydroxide
Aluminium hydroxide, Al(OH)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer polymorphs: bayerite, doyleite, and nordstrandite. Aluminium hydroxide is amphoteric, i.e., it has both basic and acidic properties. Closely related are aluminium oxide hydroxide, AlO(OH), and aluminium oxide or alumina (Al2O3), the latter of which is also amphoteric. These compounds together are the major components of the aluminium ore bauxite. Aluminium hydroxide also forms a gelatinous precipitate in water. Structure Al(OH)3 is built up of double layers of hydroxyl groups with aluminium ions occupying two-thirds of the octahedral holes between the two layers. Four polymorphs are recognized. All feature layers of octahedral aluminium hydroxide units, with hydrogen bonds between the layers. The polymorphs differ in terms of the stacking of the layers. All forms of Al(OH)3 crystals are hexagonal : *gibbsite is also known as γ-Al(OH)3 or α-Al(OH) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
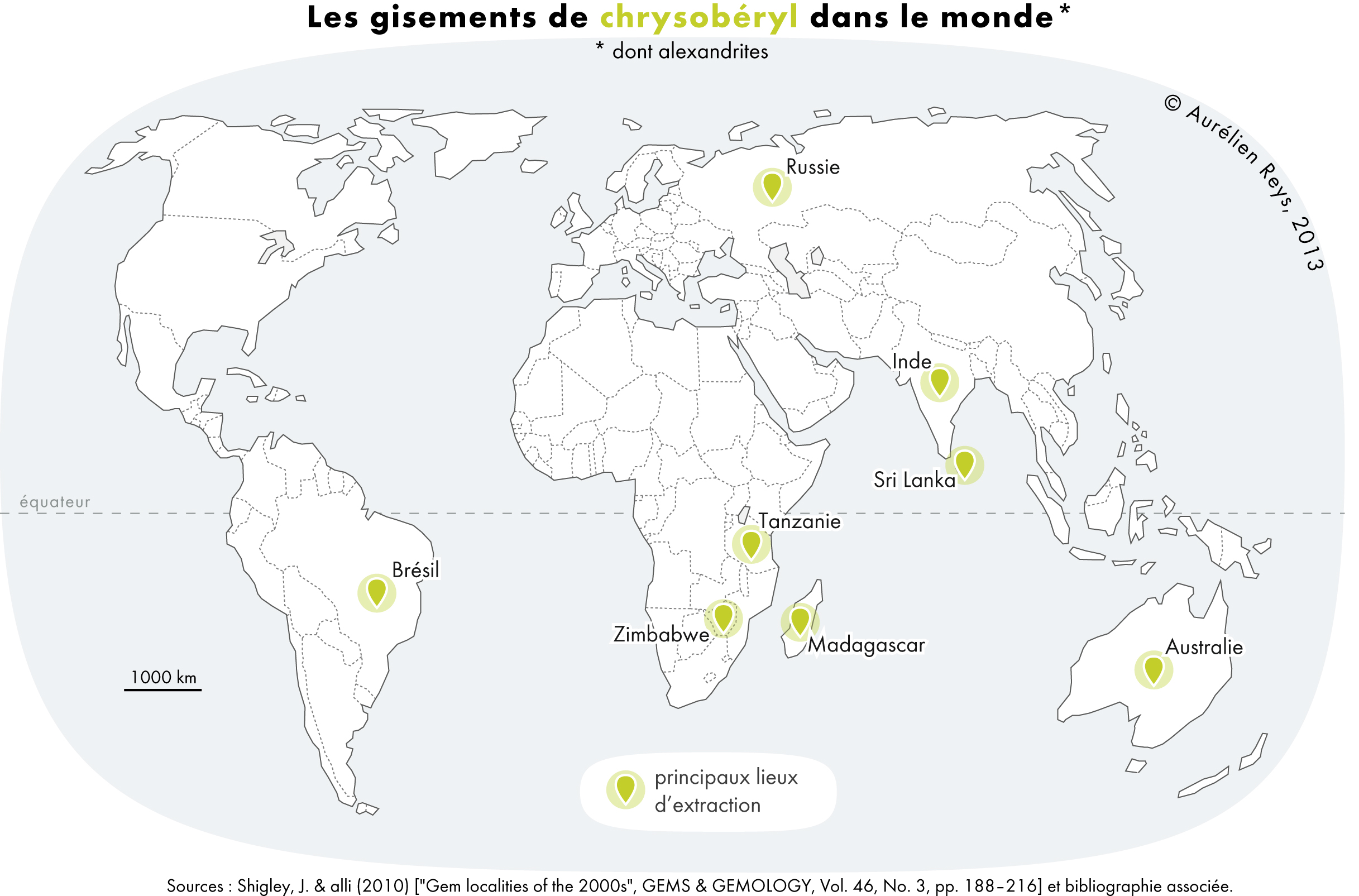
Chrysoberyl
The mineral or gemstone chrysoberyl is an aluminate of beryllium with the formula Be Al2 O4. The name chrysoberyl is derived from the Greek words χρυσός ''chrysos'' and βήρυλλος ''beryllos'', meaning "a gold-white spar". Despite the similarity of their names, chrysoberyl and beryl are two completely different gemstones, although they both contain beryllium. Chrysoberyl is the third-hardest frequently encountered natural gemstone and lies at 8.5 on the Mohs scale of mineral hardness, between corundum (9) and topaz (8). An interesting feature of its crystals are the cyclic twins called ''trillings''. These twinned crystals have a hexagonal appearance, but are the result of a triplet of twins with each "twin" oriented at 120° to its neighbors and taking up 120° of the cyclic trilling. If only two of the three possible twin orientations are present, a "V"-shaped twin results. Ordinary chrysoberyl is yellowish-green and transparent to translucent. When the mineral e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perovskite (structure)
A perovskite is any material with a crystal structure following the formula ABX3, which was first discovered as the mineral called perovskite, which consists of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). 'A' and 'B' are two positively charged ions (i.e. cations), often of very different sizes, and X is a negatively charged ion (an anion, frequently oxide) that bonds to both cations. The 'A' atoms are generally larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. Additional perovskite forms may exist where either/both the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configuration as ions within the A and B sites undergo changes in thei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |