ribose on:
[Wikipedia]
[Google]
[Amazon]
 Ribose is a
Ribose is a
File:Alpha-D-Ribopyranose numbered.png, α--Ribopyranose
File:Beta-D-Ribopyranose numbered.png, β--Ribopyranose
File:Alpha-D-Ribofuranose numbered.png, α--Ribofuranose
File:Beta-D-Ribofuranose Numbered.png, β--Ribofuranose
Relative abundance of forms of ribose in solution: β--ribopyranose (59%), α--ribopyranose (20%), β--ribofuranose (13%), α--ribofuranose (7%) and open chain (0.1%).
For ribose residues in
File:2' endo.jpg, 2' endo
File:2' endo 3' exo.jpg, 2' endo 3' exo
File:3' endo 2' exo.jpg, 3' endo 2' exo
File:3' endo.jpg, 3' endo
A ribose molecule is typically represented as a planar molecule on paper. Despite this, it is typically non-planar in nature. Even between hydrogen atoms, the many constituents on a ribose molecule cause

 Ribose is a
Ribose is a simple sugar
Monosaccharides (from Greek ''monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built.
They are usually colorless, water-solub ...
and carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or m ...
with molecular formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
C5H10O5 and the linear-form composition H−(C=O)−(CHOH)4−H. The naturally-occurring form, , is a component of the ribonucleotides from which RNA is built, and so this compound is necessary for coding, decoding, regulation
Regulation is the management of complex systems according to a set of rules and trends. In systems theory, these types of rules exist in various fields of biology and society, but the term has slightly different meanings according to context. Fo ...
and expression
Expression may refer to:
Linguistics
* Expression (linguistics), a word, phrase, or sentence
* Fixed expression, a form of words with a specific meaning
* Idiom, a type of fixed expression
* Metaphorical expression, a particular word, phrase, o ...
of gene
In biology, the word gene (from , ; "... Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a b ...
s. It has a structural analog
A structural analog (analogue in modern traditional English; Commonwealth English), also known as a chemical analog or simply an analog, is a compound having a structure similar to that of another compound, but differing from it in respect to a c ...
, deoxyribose
Deoxyribose, or more precisely 2-deoxyribose, is a monosaccharide with idealized formula H−(C=O)−(CH2)−(CHOH)3−H. Its name indicates that it is a deoxy sugar, meaning that it is derived from the sugar ribose by loss of a hydroxy group. D ...
, which is a similarly essential component of DNA. is an unnatural sugar that was first prepared by Emil Fischer
Hermann Emil Louis Fischer (; 9 October 1852 – 15 July 1919) was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He also developed the Fischer projection, a symbolic way of draw ...
and Oscar Piloty
Oskar Piloty (30 April 1866 – 6 October 1915) was a German chemist.
Life
Oskar Piloty was born the son of the painter Karl von Piloty in Munich. Due to the closeness of the Piloty family to the chemist Ludwig Knorr, who later married the ...
in 1891. It was not until 1909 that Phoebus Levene
Phoebus Aaron Theodore Levene (25 February 1869 – 6 September 1940) was a Russian born American biochemist who studied the structure and function of nucleic acids. He characterized the different forms of nucleic acid, DNA from RNA, and fo ...
and Walter Jacobs
Marion Walter Jacobs (May 1, 1930 – February 15, 1968), known as Little Walter, was an American blues musician, singer, and songwriter, whose revolutionary approach to the harmonica had a strong impact on succeeding generations, earning him ...
recognised that was a natural product, the enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...
of Fischer and Piloty's product, and an essential component of nucleic acid
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main cl ...
s. Fischer chose the name "ribose" as it is a partial rearrangement of the name of another sugar, arabinose, of which ribose is an epimer
In stereochemistry, an epimer is one of a pair of diastereomers. The two epimers have opposite configuration at only one stereogenic center out of at least two. All other stereogenic centers in the molecules are the same in each. Epimerization i ...
at the 2' carbon; both names also relate to gum arabic
Gum arabic, also known as gum sudani, acacia gum, Arabic gum, gum acacia, acacia, Senegal gum, Indian gum, and by other names, is a natural gum originally consisting of the hardened sap of two species of the ''Acacia'' tree, '' Senegalia se ...
, from which arabinose was first isolated and from which they prepared .
Like most sugars, ribose exists as a mixture of cyclic forms in equilibrium with its linear form, and these readily interconvert especially in aqueous solution. The name "ribose" is used in biochemistry and biology to refer to all of these forms, though more specific names for each are used when required. In its linear form, ribose can be recognised as the pentose
In chemistry, a pentose is a monosaccharide (simple sugar) with five carbon atoms. The chemical formula of many pentoses is , and their molecular weight is 150.13 g/mol.hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydro ...
functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the re ...
s on the same side in its Fischer projection
In chemistry, the Fischer projection, devised by Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates ...
. has these hydroxyl groups on the right hand side and is associated with the systematic name A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nomenclature.
A semisystematic name or semitrivial ...
(2''R'',3''R'',4''R'')-2,3,4,5-tetrahydroxypentanal, whilst has its hydroxyl groups appear on the left hand side in a Fischer projection. Cyclisation of ribose occurs via hemiacetal
A hemiacetal or a hemiketal has the general formula R1R2C(OH)OR, where R1 or R2 is hydrogen or an organic substituent. They generally result from the addition of an alcohol to an aldehyde or a ketone, although the latter are sometimes called hemi ...
formation due to attack on the aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
by the C4' hydroxyl group to produce a furanose
A furanose is a collective term for carbohydrates that have a chemical structure that includes a five-membered ring system consisting of four carbon atoms and one oxygen atom. The name derives from its similarity to the oxygen heterocycle furan, bu ...
form or by the C5' hydroxyl group to produce a pyranose form. In each case, there are two possible geometric outcomes, named as α- and β- and known as anomers, depending on the stereochemistry at the hemiacetal carbon atom (the "anomeric carbon"). At room temperature, about 76% of is present in pyranose forms (α:β = 1:2) and 24% in the furanose forms (α:β = 1:3), with only about 0.1% of the linear form present.
The ribonucleoside A ribonucleoside is a type of nucleoside including ribose as a component.
One example of a ribonucleoside is cytidine
Cytidine (symbol C or Cyd) is a nucleoside molecule that is formed when cytosine is attached to a ribose ring (also known as a ...
s adenosine, cytidine
Cytidine (symbol C or Cyd) is a nucleoside molecule that is formed when cytosine is attached to a ribose ring (also known as a ribofuranose) via a β-N1- glycosidic bond. Cytidine is a component of RNA. It is a white water-soluble solid. which ...
, guanosine
Guanosine (symbol G or Guo) is a purine nucleoside comprising guanine attached to a ribose ( ribofuranose) ring via a β-N9- glycosidic bond. Guanosine can be phosphorylated to become guanosine monophosphate (GMP), cyclic guanosine monophosphate ...
, and uridine
Uridine (symbol U or Urd) is a glycosylated pyrimidine analog containing uracil attached to a ribose ring (or more specifically, a ribofuranose) via a β-N1-glycosidic bond. The analog is one of the five standard nucleosides which make up nucle ...
are all derivative
In mathematics, the derivative of a function of a real variable measures the sensitivity to change of the function value (output value) with respect to a change in its argument (input value). Derivatives are a fundamental tool of calculus. ...
s of β--ribofuranose. Metabolically-important species that include phosphorylated
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, wh ...
ribose include ADP, ATP, coenzyme A, and NADH
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an aden ...
. cAMP
Camp may refer to:
Outdoor accommodation and recreation
* Campsite or campground, a recreational outdoor sleeping and eating site
* a temporary settlement for nomads
* Camp, a term used in New England, Northern Ontario and New Brunswick to descri ...
and cGMP serve as secondary messengers in some signaling pathways and are also ribose derivatives. The ribose moiety
Moiety may refer to:
Chemistry
* Moiety (chemistry), a part or functional group of a molecule
** Moiety conservation, conservation of a subgroup in a chemical species
Anthropology
* Moiety (kinship), either of two groups into which a society is ...
appears in some pharmaceutical agents, including the antibiotics neomycin
Neomycin is an aminoglycoside antibiotic that displays bactericidal activity against gram-negative aerobic bacilli and some anaerobic bacilli where resistance has not yet arisen. It is generally not effective against gram-positive bacilli and ...
and paromomycin
Paromomycin is an antimicrobial used to treat a number of parasitic infections including amebiasis, giardiasis, leishmaniasis, and tapeworm infection. It is a first-line treatment for amebiasis or giardiasis during pregnancy. Otherwise it is g ...
.
Synthesis and sources
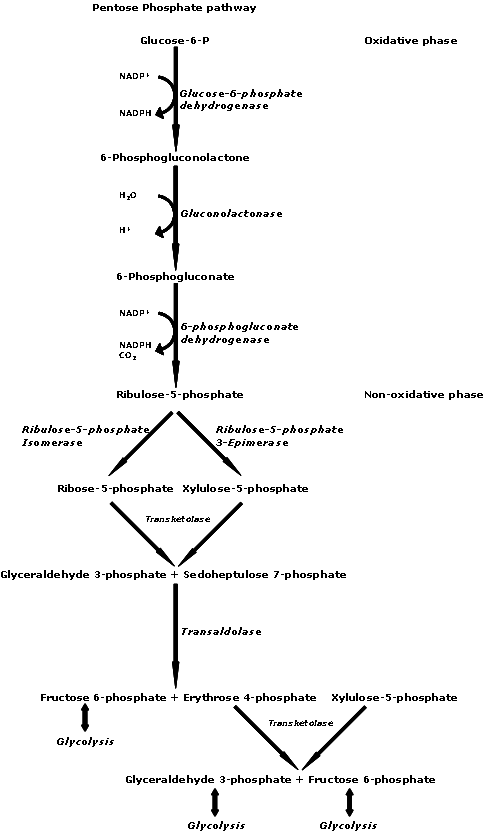
Ribose as its 5-phosphate ester is typically produced from glucose by thepentose phosphate pathway
The pentose phosphate pathway (also called the phosphogluconate pathway and the hexose monophosphate shunt and the HMP Shunt) is a metabolic pathway parallel to glycolysis. It generates NADPH and pentoses (5-carbon sugars) as well as ribose 5-pho ...
. In at least some archaea, alternative pathways have been identified.
Ribose can be synthesized chemically, but commercial production relies on fermentation of glucose. Using genetically modified strains of ''B. subtilis
''Bacillus subtilis'', known also as the hay bacillus or grass bacillus, is a Gram-positive, catalase-positive bacterium, found in soil and the gastrointestinal tract of ruminants, humans and marine sponges. As a member of the genus ''Bacillu ...
'', 90 g/liter of ribose can be produced from 200 g of glucose. The conversion entails the intermediacy of gluconate and ribulose.
Ribose has been detected in meteorite
A meteorite is a solid piece of debris from an object, such as a comet, asteroid, or meteoroid, that originates in outer space and survives its passage through the atmosphere to reach the surface of a planet or Natural satellite, moon. When the ...
s.
Structure
Ribose is an aldopentose (a monosaccharide containing fivecarbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
atoms that, in its open chain
In chemistry, an open-chain compound (also spelled as open chain compound) or acyclic compound (Greek prefix "α", ''without'' and "κύκλος", ''cycle'') is a compound with a linear structure, rather than a cyclic one.
An open-chain compound h ...
form, has an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the re ...
at one end). In the conventional numbering scheme for monosaccharides, the carbon atoms are numbered from C1' (in the aldehyde group) to C5'. The deoxyribose
Deoxyribose, or more precisely 2-deoxyribose, is a monosaccharide with idealized formula H−(C=O)−(CH2)−(CHOH)3−H. Its name indicates that it is a deoxy sugar, meaning that it is derived from the sugar ribose by loss of a hydroxy group. D ...
derivative found in DNA differs from ribose by having a hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
atom in place of the hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydro ...
group at C2'. This hydroxyl group performs a function in RNA splicing.
The "-" in the name -ribose refers to the stereochemistry of the chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
carbon atom farthest away from the aldehyde group (C4'). In -ribose, as in all -sugars, this carbon atom has the same configuration as in -glyceraldehyde.nucleoside
Nucleosides are glycosylamines that can be thought of as nucleotides without a phosphate group. A nucleoside consists simply of a nucleobase (also termed a nitrogenous base) and a five-carbon sugar (ribose or 2'-deoxyribose) whereas a nucleoti ...
s and nucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecule ...
, the torsion angles for the rotation encompassing the bonds influence the configuration of the respective nucleoside and nucleotide. The secondary structure
Protein secondary structure is the three dimensional conformational isomerism, form of ''local segments'' of proteins. The two most common Protein structure#Secondary structure, secondary structural elements are alpha helix, alpha helices and beta ...
of a nucleic acid is determined by the rotation of its 7 torsion angle
A dihedral angle is the angle between two intersecting planes or half-planes. In chemistry, it is the clockwise angle between half-planes through two sets of three atoms, having two atoms in common. In solid geometry, it is defined as the uni ...
s. Having a large amount of torsion angles allows for greater flexibility.
In closed ring riboses, the observed flexibility mentioned above is not observed because the ring cycle imposes a limit on the number of torsion angles possible in the structure. Conformers of closed form riboses differ in regards to how the lone oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
in the molecule is positioned respective to the nitrogenous base
Nucleobases, also known as ''nitrogenous bases'' or often simply ''bases'', are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic b ...
(also known as a nucleobase
Nucleobases, also known as ''nitrogenous bases'' or often simply ''bases'', are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic b ...
or just a base) attached to the ribose. If a carbon is facing towards the base, then the ribose is labeled as endo. If a carbon is facing away from the base, then the ribose is labeled as exo. If there is an oxygen molecule attached to the 2' carbon of a closed cycle ribose, then the exo confirmation is more stable because it decreases the interactions of the oxygen with the base. The difference itself is quite small, but when looking at an entire chain of RNA the slight difference amounts to a sizable impact.steric hindrance
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
and strain between them. To relieve this crowding and ring strain, the ring puckers, i.e. becomes non-planar. This puckering is achieved by displacing an atom from the plane, relieving the strain and yielding a more stable configuration. Puckering, otherwise known as the sugar ring conformation (specifically ribose sugar), can be described by the amplitude of pucker as well as the pseudorotation
In chemistry, a pseudorotation is a set of intramolecular movements of attached groups (i.e., ligands) on a highly symmetric molecule, leading to a molecule indistinguishable from the initial one. The International Union of Pure and Applied Chem ...
angle. The pseudo-rotation angle can be described as either "north (N)" or "south (S)" range. While both ranges are found in double helices, the north range is commonly associated with RNA and the A form of DNA. In contrast, the south range is associated with B form DNA. Z-DNA
Z-DNA is one of the many possible double helical structures of DNA. It is a left-handed double helical structure in which the helix winds to the left in a zigzag pattern, instead of to the right, like the more common B-DNA form. Z-DNA is thought ...
contains sugars in both the north and south ranges. When only a single atom is displaced, it is referred to as an "envelope" pucker. When two atoms are displaced, it is referred to as a "twist" pucker, in reference to the zigzag orientation. In an "endo" pucker, the major displacement of atoms is on the β-face, the same side as the C4'-C5' bond and the base. In an "exo" pucker, the major displacement of atoms is on the α-face, on the opposite side of the ring. The major forms of ribose are the 3'-endo pucker (commonly adopted by RNA and A-form DNA) and 2'-endo pucker (commonly adopted by B-form DNA). These ring puckers are developed from changes in ring torsion angles; there are infinite combinations of angles so therefore, there is an infinite number of transposable pucker conformations, each separated by disparate activation energies.
Functions
ATP is derived from ribose; it contains one ribose, threephosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phospho ...
groups, and an adenine
Adenine () ( symbol A or Ade) is a nucleobase (a purine derivative). It is one of the four nucleobases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The three others are guanine, cytosine and thymine. Its deri ...
base. ATP is created during cellular respiration
Cellular respiration is the process by which biological fuels are oxidised in the presence of an inorganic electron acceptor such as oxygen to produce large amounts of energy, to drive the bulk production of ATP. Cellular respiration may be des ...
from adenosine diphosphate (ATP with one less phosphate group).
Signaling pathways
Ribose is a building block in secondary signaling molecules such ascyclic adenosine monophosphate
Cyclic adenosine monophosphate (cAMP, cyclic AMP, or 3',5'-cyclic adenosine monophosphate) is a second messenger important in many biological processes. cAMP is a derivative of adenosine triphosphate (ATP) and used for intracellular signal transd ...
(cAMP) which is derived from ATP. One specific case in which cAMP is used is in cAMP-dependent signaling pathways. In cAMP signaling pathways, either a stimulative or inhibitory hormone receptor is activated by a signal molecule
In biology, cell signaling (cell signalling in British English) or cell communication is the ability of a cell to receive, process, and transmit signals with its environment and with itself. Cell signaling is a fundamental property of all cellula ...
. These receptors are linked to a stimulative or inhibitory regulative G-protein
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their act ...
. When a stimulative G-protein is activated, adenylyl cyclase
Adenylate cyclase (EC 4.6.1.1, also commonly known as adenyl cyclase and adenylyl cyclase, abbreviated AC) is an enzyme with systematic name ATP diphosphate-lyase (cyclizing; 3′,5′-cyclic-AMP-forming). It catalyzes the following reaction:
:A ...
catalyzes ATP into cAMP by using Mg2+ or Mn2+. cAMP, a secondary messenger, then goes on to activate protein kinase A
In cell biology, protein kinase A (PKA) is a family of enzymes whose activity is dependent on cellular levels of cyclic AMP (cAMP). PKA is also known as cAMP-dependent protein kinase (). PKA has several functions in the cell, including regulatio ...
, which is an enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
that regulates cell metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run c ...
. Protein kinase A regulates metabolic enzymes by phosphorylation
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, wh ...
which causes a change in the cell depending on the original signal molecule. The opposite occurs when an inhibitory G-protein is activated; the G-protein inhibits adenylyl cyclase and ATP is not converted to cAMP. 
Metabolism
Ribose is referred to as the "molecular currency" because of its involvement in intracellular energy transfers. For example, nicotinamide adenine dinucleotide (NAD),flavin adenine dinucleotide
Flavin may refer to:
Placename
* Flavin, Aveyron, a commune in southern France
Surname
* Adrian Flavin (born 1979), a professional rugby player
* Christopher Flavin, president of the Worldwatch Institute
* Dan Flavin (1933–1996), a minimalis ...
(FAD), and nicotinamide adenine dinucleotide phosphate
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NAD ...
(NADP) all contain the -ribofuranose moiety
Moiety may refer to:
Chemistry
* Moiety (chemistry), a part or functional group of a molecule
** Moiety conservation, conservation of a subgroup in a chemical species
Anthropology
* Moiety (kinship), either of two groups into which a society is ...
. They can each be derived from -ribose after it is converted to -ribose 5-phosphate by the enzyme ribokinase
In enzymology, a ribokinase () is an enzyme that catalyzes the chemical reaction
:ATP + -ribose ⇌ ADP + -ribose 5-phosphate
Thus, the two substrates of this enzyme are ATP and -ribose, whereas its two products are ADP and -ribose 5-phosp ...
. NAD, FAD, and NADP act as electron acceptors in biochemical redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
reactions in major metabolic pathways including glycolysis, the citric acid cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and protein ...
, fermentation, and the electron transport chain.

Nucleotide biosynthesis
Nucleotides are synthesized through salvage orde novo synthesis
In chemistry, ''de novo'' synthesis () refers to the synthesis of complex molecules from simple molecules such as sugars or amino acids, as opposed to recycling after partial degradation. For example, nucleotides are not needed in the diet as ...
. Nucleotide salvage A salvage pathway is a pathway in which a biological product is produced from intermediates in the degradative pathway of its own or a similar substance. The term often refers to nucleotide salvage in particular, in which nucleotides (purine and py ...
uses pieces of previously made nucleotides and re-synthesizes them for future use. In de novo, amino acids, carbon dioxide, folate derivatives, and phosphoribosyl pyrophosphate
Phosphoribosyl pyrophosphate (PRPP) is a pentose phosphate. It is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, as well as in pyrimidine nucleotide formation. Hence it is a building block for DN ...
(PRPP) are used to synthesize nucleotides. Both de novo and salvage require PRPP which is synthesized from ATP and ribose 5-phosphate by an enzyme called PRPP synthetase
Ribose-phosphate diphosphokinase (or phosphoribosyl pyrophosphate synthetase or ribose-phosphate pyrophosphokinase) is an enzyme that converts ribose 5-phosphate into phosphoribosyl pyrophosphate (PRPP). It is classified under .
The enzyme is ...
.
Modifications
Modifications in nature
Ribokinase
In enzymology, a ribokinase () is an enzyme that catalyzes the chemical reaction
:ATP + -ribose ⇌ ADP + -ribose 5-phosphate
Thus, the two substrates of this enzyme are ATP and -ribose, whereas its two products are ADP and -ribose 5-phosp ...
catalyzes the conversion of -ribose to -ribose 5-phosphate. Once converted, -ribose-5-phosphate is available for the manufacturing of the amino acids tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α- carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic ...
and histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the d ...
, or for use in the pentose phosphate pathway
The pentose phosphate pathway (also called the phosphogluconate pathway and the hexose monophosphate shunt and the HMP Shunt) is a metabolic pathway parallel to glycolysis. It generates NADPH and pentoses (5-carbon sugars) as well as ribose 5-pho ...
. The absorption of -ribose is 88–100% in the small intestines (up to 200 mg/kg·h).
One important modification occurs at the C2' position of the ribose molecule. By adding an O-alkyl group, the nuclear resistance of the RNA is increased because of additional stabilizing forces. These forces are stabilizing because of the increase of intramolecular hydrogen bonding and an increase in the glycosidic bond
A glycosidic bond or glycosidic linkage is a type of covalent bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.
A glycosidic bond is formed between the hemiacetal or hemiketal group ...
stability. The resulting increase of resistance leads to increases in the half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
of siRNA
Small interfering RNA (siRNA), sometimes known as short interfering RNA or silencing RNA, is a class of double-stranded RNA at first non-coding RNA molecules, typically 20-24 (normally 21) base pairs in length, similar to miRNA, and operating ...
and the potential therapeutic potential in cells and animals. The methylation of ribose at particular sites is correlated with a decrease in immune stimulation.
Synthetic modifications
Along with phosphorylation, ribofuranose molecules can exchange their oxygen withselenium
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, ...
and sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
to produce similar sugars that only vary at the 4' position. These derivatives are more lipophilic
Lipophilicity (from Greek λίπος "fat" and φίλος "friendly"), refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such non-polar solvents are themselves lipo ...
than the original molecule. Increased lipophilicity makes these species more suitable for use in techniques such as PCR, RNA aptamer post-modification, antisense technology, and for phasing X-ray crystallographic data.
Similar to the 2' modifications in nature, a synthetic modification of ribose includes the addition of fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
at the 2' position. This fluorinated
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a chemical compound, compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the prod ...
ribose acts similar to the methylated ribose because it is capable of suppressing immune stimulation depending on the location of the ribose in the DNA strand. The big difference between methylation and fluorination, is the latter only occurs through synthetic modifications. The addition of fluorine leads to an increase in the stabilization of the glycosidic bond and an increase of intramolecular hydrogen bonds.
Medical uses
-ribose has been suggested for use in management ofcongestive heart failure
Heart failure (HF), also known as congestive heart failure (CHF), is a syndrome, a group of signs and symptoms caused by an impairment of the heart's blood pumping function. Symptoms typically include shortness of breath, excessive fatigue, ...
(as well as other forms of heart disease) and for chronic fatigue syndrome (CFS), also called myalgic encephalomyelitis (ME) in an open-label non-blinded, non-randomized, and non-crossover subjective study.
Supplemental -ribose can bypass part of the pentose phosphate pathway
The pentose phosphate pathway (also called the phosphogluconate pathway and the hexose monophosphate shunt and the HMP Shunt) is a metabolic pathway parallel to glycolysis. It generates NADPH and pentoses (5-carbon sugars) as well as ribose 5-pho ...
, an energy-producing pathway, to produce -ribose-5-phosphate. The enzyme glucose-6-phosphate-dehydrogenase (G-6-PDH) is often in short supply in cells, but more so in diseased tissue, such as in myocardial
Cardiac muscle (also called heart muscle, myocardium, cardiomyocytes and cardiac myocytes) is one of three types of vertebrate muscle tissues, with the other two being skeletal muscle and smooth muscle. It is an involuntary, striated muscle that ...
cells in patients with cardiac disease. The supply of -ribose in the mitochondria is directly correlated with ATP production; decreased -ribose supply reduces the amount of ATP being produced. Studies suggest that supplementing -ribose following tissue ischemia (e.g. myocardial ischemia) increases myocardial ATP production, and therefore mitochondrial function. Essentially, administering supplemental -ribose bypasses an enzymatic step in the pentose phosphate pathway by providing an alternate source of 5-phospho--ribose 1-pyrophosphate
In chemistry, pyrophosphates are phosphorus oxyanions that contain two phosphorus atoms in a P–O–P linkage. A number of pyrophosphate salts exist, such as disodium pyrophosphate (Na2H2P2O7) and tetrasodium pyrophosphate (Na4P2O7), among othe ...
for ATP production. Supplemental -ribose enhances recovery of ATP levels while also reducing cellular injury in humans and other animals. One study suggested that the use of supplemental -ribose reduces the instance of angina in men with diagnosed coronary artery disease. -Ribose has been used to treat many pathological
Pathology is the study of the causes and effects of disease or injury. The word ''pathology'' also refers to the study of disease in general, incorporating a wide range of biology research fields and medical practices. However, when used in th ...
conditions, such as chronic fatigue syndrome, fibromyalgia, and myocardial dysfunction. It is also used to reduce symptoms of cramping, pain, stiffness, etc. after exercise and to improve athletic performance.
References