Plastoquinone on:
[Wikipedia]
[Google]
[Amazon]
Plastoquinone (PQ) is an isoprenoid quinone molecule involved in the electron transport chain in the 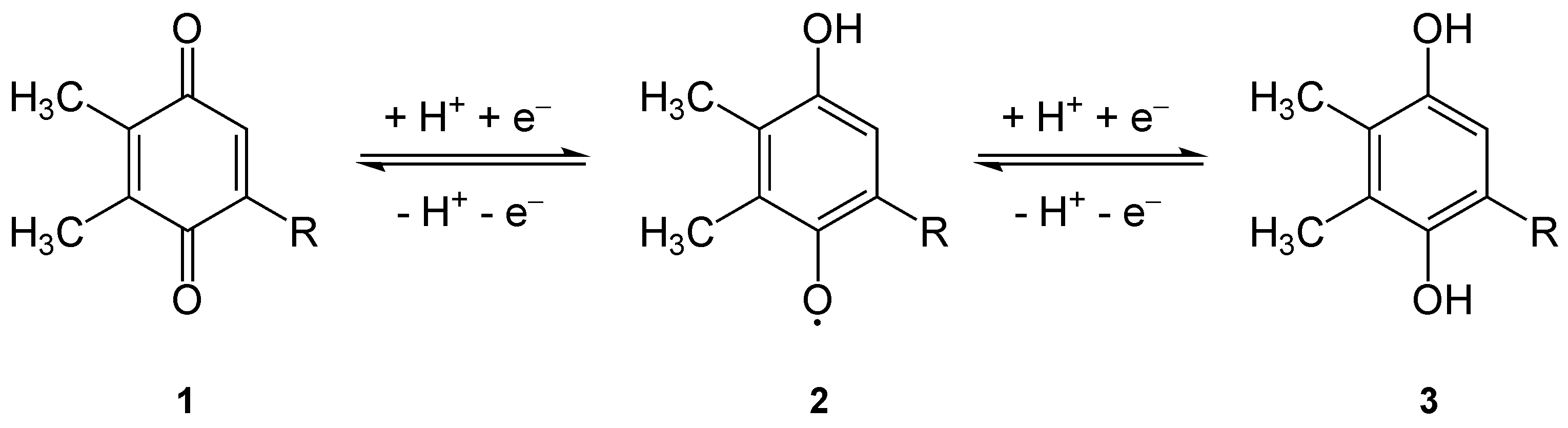 The prefix ''plasto-'' means either plastid or chloroplast, alluding to its location within the cell.
The prefix ''plasto-'' means either plastid or chloroplast, alluding to its location within the cell.
 The role that plastoquinone plays in photosynthesis, more specifically in the light-dependent reactions of photosynthesis, is that of a mobile electron carrier through the membrane of the
The role that plastoquinone plays in photosynthesis, more specifically in the light-dependent reactions of photosynthesis, is that of a mobile electron carrier through the membrane of the

Plastoquinones
History, absorption spectra, and analogs. Photosynthesis Light reactions 1,4-Benzoquinones Terpenes and terpenoids
light-dependent reaction
Light-dependent reactions is jargon for certain photochemical reactions that are involved in photosynthesis, the main process by which plants acquire energy. There are two light dependent reactions, the first occurs at photosystem II (PSII) and ...
s of photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
. The most common form of plastoquinone, known as PQ-A or PQ-9, is a 2,3-dimethyl-1,4-benzoquinone Benzoquinone (C6H4O2) is a quinone with a single benzene ring. There are 2 (out of 3 hypothetical) benzoquinones:
* 1,4-Benzoquinone, most commonly, right image (also ''para''-benzoquinone, ''p''-benzoquinone, ''para''-quinone, or just quinone)
* 1 ...
molecule with a side chain of nine isoprenyl units. There are other forms of plastoquinone, such as ones with shorter side chains like PQ-3 (which has 3 isoprenyl side units instead of 9) as well as analogs such as PQ-B, PQ-C, and PQ-D, which differ in their side chains. The benzoquinone and isoprenyl units are both nonpolar
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end.
Polar molecules must contain one or more polar ...
, anchoring the molecule within the inner section of a lipid bilayer
The lipid bilayer (or phospholipid bilayer) is a thin polar membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around all cells. The cell membranes of almost all organisms and many vir ...
, where the hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, t ...
tails are usually found.
Plastoquinones are very structurally similar to ubiquinone, or coenzyme Q10, differing by the length of the isoprenyl side chain, replacement of the methoxy groups with methyl groups, and removal of the methyl group in the 2 position on the quinone. Like ubiquinone, it can come in several oxidation states: plastoquinone, plastosemiquinone (unstable), and plastoquinol, which differs from plastoquinone by having two hydroxyl groups
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
instead of two carbonyl group
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
s.
Plastoquinol, the reduced form, also functions as an antioxidant by reducing reactive oxygen species
In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (). Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.
The reduction of molecular oxygen () p ...
, some produced from the photosynthetic reactions, that could harm the cell membrane. One example of how it does this is by reacting with superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of t ...
s to form hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
and plastosemiquinone.
 The prefix ''plasto-'' means either plastid or chloroplast, alluding to its location within the cell.
The prefix ''plasto-'' means either plastid or chloroplast, alluding to its location within the cell.
Role in photosynthesis
thylakoid
Thylakoids are membrane-bound compartments inside chloroplasts and cyanobacteria. They are the site of the light-dependent reactions of photosynthesis. Thylakoids consist of a thylakoid membrane surrounding a thylakoid lumen. Chloroplast thyl ...
.
Plastoquinone is reduced when it accepts two electrons from photosystem II and two hydrogen cations (H+) from the stroma of the chloroplast, thereby forming plastoquinol (PQH2). It transfers the electrons further down the electron transport chain to plastocyanin
Plastocyanin is a copper-containing protein that mediates electron-transfer. It is found in a variety of plants, where it participates in photosynthesis. The protein is a prototype of the blue copper proteins, a family of intensely blue-colored ...
, a mobile, water-soluble electron carrier, through the cytochrome ''b''6''f'' protein complex. The cytochrome ''b''6''f'' protein complex catalyzes the electron transfer between plastoquinone and plastocyanin, but also transports the two protons into the lumen of thylakoid discs. This proton transfer forms an electrochemical gradient, which is used by ATP synthase at the end of the light dependent reactions in order to form ATP from ADP and Pi.
Within photosystem II
Plastoquinone is found within photosystem II in two specific binding sites, known as QA and QB. The plastoquinone at QA, the primary binding site, is very tightly bound, compared to the plastoquinone at QB, the secondary binding site, which is much more easily removed. QA is only transferred a single electron, so it has to transfer an electron to QB twice before QB is able to pick up two protons from the stroma and be replaced by another plastoquinone molecule. The protonated QB then joins a pool of free plastoquinone molecules in the membrane of the thylakoid. The free plastoquinone molecules eventually transfer electrons to the water-soluble plastocyanin so as to continue the light-dependent reactions. There are additional plastoquinone binding sites within photosystem II (QC and possibly QD), but their function and/or existence have not been fully elucidated.Biosynthesis
The p-hydroxyphenylpyruvate is synthesized fromtyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the G ...
, while the solanesyl diphosphate is synthesized through the MEP/DOXP pathway. Homogentisate is formed from p-hydroxyphenylpyruvate and is then combined with solanesyl diphosphate through a condensation reaction
In organic chemistry, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule such as water. If water is lost, the reaction is also known as a ...
. The resulting intermediate, 2-methyl-6-solanesyl-1,4-benzoquinol is then methylated
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These ...
to form the final product, plastoquinol-9. This pathway is used in most photosynthetic organisms, like algae and plants. However, cyanobacteria appear to not use homogentisate for synthesizing plastoquinol, possibly resulting in a pathway different from the one shown below.
Derivatives
Some derivatives that were designed to penetrate mitochondrial cell membranes ( SkQ1 (plastoquinonyl-decyl-triphenylphosphonium), SkQR1 (therhodamine
Rhodamine is a family of related dyes, a subset of the triarylmethane dyes. They are derivatives of xanthene. Important members of the rhodamine family are Rhodamine 6G, Rhodamine 123, and Rhodamine B. They are mainly used to dye paper and inks ...
-containing analog of SkQ1), SkQ3) have anti-oxidant and protonophore activity. SkQ1 has been proposed as an anti-aging treatment, with the possible reduction of age-related vision issues due to its antioxidant ability. This antioxidant ability results from both its antioxidant ability to reduce reactive oxygen species (derived from the part of the molecule containing plastoquinonol), which are often formed within mitochondria, as well as its ability to increase ion exchange across membranes (derived from the part of the molecule containing cations that can dissolve within membranes). Specifically, like plastoquinol, SkQ1 has been shown to scavenge superoxides both within cells (in vivo) and outside of cells (in vitro). SkQR1 and SkQ1 have also been proposed as a possible way to treat brain issues like Alzheimer's due to their ability to potentially fix damages caused by amyloid beta. Additionally, SkQR1 has been shown as a way to reduce the issues caused by brain trauma through its antioxidant abilities, which help prevent cell death signals by reducing the amounts of reactive oxygen species coming from mitochondria.
References
{{reflistExternal links
Plastoquinones
History, absorption spectra, and analogs. Photosynthesis Light reactions 1,4-Benzoquinones Terpenes and terpenoids