monosaccharide on:
[Wikipedia]
[Google]
[Amazon]
Monosaccharides (from
 The - and - prefixes are also used with other monosaccharides, to distinguish two particular stereoisomers that are mirror-images of each other. For this purpose, one considers the chiral carbon that is furthest removed from the C=O group. Its four bonds must connect to −H, −OH, −C(OH)H, and the rest of the molecule. If the molecule can be rotated in space so that the directions of those four groups match those of the analog groups in -glyceraldehyde's C2, then the isomer receives the - prefix. Otherwise, it receives the - prefix.
In the Fischer projection, the - and - prefixes specifies the configuration at the carbon atom that is second from bottom: - if the hydroxyl is on the right side, and - if it is on the left side.
Note that the - and - prefixes do not indicate the direction of rotation of polarized light, which is a combined effect of the arrangement at all chiral centers. However, the two enantiomers will always rotate the light in opposite directions, by the same amount. See also system.
The - and - prefixes are also used with other monosaccharides, to distinguish two particular stereoisomers that are mirror-images of each other. For this purpose, one considers the chiral carbon that is furthest removed from the C=O group. Its four bonds must connect to −H, −OH, −C(OH)H, and the rest of the molecule. If the molecule can be rotated in space so that the directions of those four groups match those of the analog groups in -glyceraldehyde's C2, then the isomer receives the - prefix. Otherwise, it receives the - prefix.
In the Fischer projection, the - and - prefixes specifies the configuration at the carbon atom that is second from bottom: - if the hydroxyl is on the right side, and - if it is on the left side.
Note that the - and - prefixes do not indicate the direction of rotation of polarized light, which is a combined effect of the arrangement at all chiral centers. However, the two enantiomers will always rotate the light in opposite directions, by the same amount. See also system.
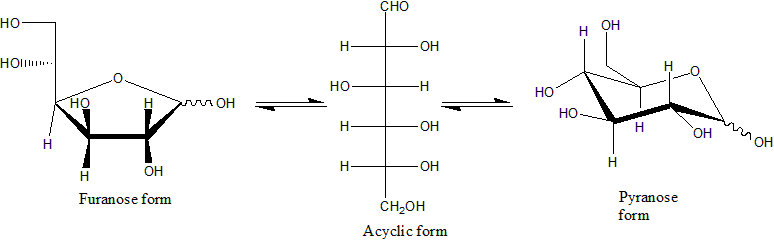
 For many monosaccharides (including glucose), the cyclic forms predominate, in the solid state and in solutions, and therefore the same name commonly is used for the open- and closed-chain isomers. Thus, for example, the term "glucose" may signify glucofuranose, glucopyranose, the open-chain form, or a mixture of the three.
Cyclization creates a new
For many monosaccharides (including glucose), the cyclic forms predominate, in the solid state and in solutions, and therefore the same name commonly is used for the open- and closed-chain isomers. Thus, for example, the term "glucose" may signify glucofuranose, glucopyranose, the open-chain form, or a mixture of the three.
Cyclization creates a new
Alpha-D-Glucopyranose.svg, α--Glucopyranose
Beta-D-Glucopyranose.svg, β--Glucopyranose
Nomenclature of Carbohydrates
{{Authority control Carbohydrate chemistry
Greek
Greek may refer to:
Greece
Anything of, from, or related to Greece, a country in Southern Europe:
*Greeks, an ethnic group.
*Greek language, a branch of the Indo-European language family.
**Proto-Greek language, the assumed last common ancestor ...
''monos
Monos is an island in the Republic of Trinidad and Tobago. It is one of the "Bocas Islands", which lie in the '' Bocas del Dragón'' (''Dragons' Mouth'') between Trinidad and Venezuela. It is so named as the island was once home to noisy red h ...
'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double ...
and the most basic units (monomer
In chemistry, a monomer ( ; '' mono-'', "one" + ''-mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
s) from which all carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or ma ...
s are built.
They are usually colorless, water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
-soluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubi ...
, and crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macro ...
line solids. Contrary to their name (sugars), only some monosaccharides have a sweet taste. Most monosaccharides have the formula (though not all molecules with this formula are monosaccharides).
Examples of monosaccharides include glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, u ...
(dextrose), fructose (levulose), and galactose
Galactose (, '' galacto-'' + ''-ose'', "milk sugar"), sometimes abbreviated Gal, is a monosaccharide sugar that is about as sweet as glucose, and about 65% as sweet as sucrose. It is an aldohexose and a C-4 epimer of glucose. A galactose molecu ...
. Monosaccharides are the building blocks of disaccharide
A disaccharide (also called a double sugar or ''biose'') is the sugar formed when two monosaccharides are joined by glycosidic linkage. Like monosaccharides, disaccharides are simple sugars soluble in water. Three common examples are sucrose, la ...
s (such as sucrose and lactose) and polysaccharides (such as cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell w ...
and starch). The table sugar
White sugar, also called table sugar, granulated sugar, or regular sugar, is a commonly used type of sugar, made either of beet sugar or cane sugar, which has undergone a refining process.
Description
The refining process completely removes ...
used in everyday vernacular is itself a disaccharide sucrose comprising one molecule of each of the two monosaccharides D-glucose and D-fructose.
Each carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
atom that supports a hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydro ...
group is chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
, except those at the end of the chain. This gives rise to a number of isomeric forms, all with the same chemical formula. For instance, galactose and glucose are both aldohexoses, but have different physical structures and chemical properties.
The monosaccharide glucose plays a pivotal role in metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run c ...
, where the chemical energy is extracted through glycolysis and the citric acid cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and protein ...
to provide energy to living organisms.
Structure and nomenclature
With few exceptions (e.g.,deoxyribose
Deoxyribose, or more precisely 2-deoxyribose, is a monosaccharide with idealized formula H−(C=O)−(CH2)−(CHOH)3−H. Its name indicates that it is a deoxy sugar, meaning that it is derived from the sugar ribose by loss of a hydroxy group. D ...
), monosaccharides have this chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
: (CH2O)''x'', where conventionally ''x'' ≥ 3. Monosaccharides can be classified by the number ''x'' of carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
atoms they contain: triose
A triose is a monosaccharide, or simple sugar, containing three carbon atoms. There are only three possible trioses (including dihydroxyacetone): L-glyceraldehyde and D-glyceraldehyde, the two enantiomers of glyceraldehyde, which are aldotrios ...
(3), tetrose A tetrose is a monosaccharide with 4 carbon atoms. They have either an aldehyde functional group in position 1 (aldotetroses) or a ketone functional group in position 2 (ketotetroses).
File:DErythrose Fischer.svg , D-Erythrose
File:DThreose Fisch ...
(4), pentose
In chemistry, a pentose is a monosaccharide (simple sugar) with five carbon atoms. The chemical formula of many pentoses is , and their molecular weight is 150.13 g/mol.hexose
In chemistry, a hexose is a monosaccharide (simple sugar) with six carbon atoms. The chemical formula for all hexoses is C6H12O6, and their molecular weight is 180.156 g/mol.
Hexoses exist in two forms, open-chain or cyclic, that easily convert ...
(6), heptose
A heptose is a monosaccharide with seven carbon atoms.
They have either an aldehyde functional group in position 1 (aldoheptoses) or a ketone functional group in position 2, 3 or 4 (ketoheptoses). Ketoheptoses have 4 chiral centers, whereas ald ...
(7), and so on.
Glucose, used as an energy source and for the synthesis of starch, glycogen and cellulose, is a hexose
In chemistry, a hexose is a monosaccharide (simple sugar) with six carbon atoms. The chemical formula for all hexoses is C6H12O6, and their molecular weight is 180.156 g/mol.
Hexoses exist in two forms, open-chain or cyclic, that easily convert ...
. Ribose and deoxyribose (in RNA
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydra ...
and DNA, respectively) are pentose sugars. Examples of heptoses include the ketose
A ketose is a monosaccharide containing one ketone group per molecule. The simplest ketose is dihydroxyacetone, which has only three carbon atoms. It is the only ketose with no optical activity. All monosaccharide ketoses are reducing sugars, be ...
s, mannoheptulose
Mannoheptulose is a heptose, a monosaccharide with seven carbon atoms, and a ketose, with the characteristic carbonyl group of the carbohydrate present on a secondary carbon (functioning as a ketone group). The sugar alcohol form of mannoheptul ...
and sedoheptulose
Sedoheptulose or pseudoheptulose or D-''altro''-heptulose is a ketoheptose—a monosaccharide with seven carbon atoms and a ketone functional group. It is one of the few heptoses found in nature, and is found in various fruits and vegetabl ...
. Monosaccharides with eight or more carbons are rarely observed as they are quite unstable. In aqueous solutions monosaccharides exist as rings if they have more than four carbons.
Linear-chain monosaccharides
Simple monosaccharides have a linear and unbranched carbon skeleton with onecarbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
(C=O) functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the re ...
, and one hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydro ...
(OH) group on each of the remaining carbon atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, ...
s. Therefore, the molecular structure of a simple monosaccharide can be written as H(CHOH)''n''(C=O)(CHOH)''m''H, where ''n'' + 1 + ''m'' = ''x''; so that its elemental formula is C''x''H2''x''O''x''.
By convention, the carbon atoms are numbered from 1 to ''x'' along the backbone, starting from the end that is closest to the C=O group. Monosaccharides are the simplest units of carbohydrates and the simplest form of sugar.
If the carbonyl is at position 1 (that is, ''n'' or ''m'' is zero), the molecule begins with a formyl group H(C=O)− and is technically an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
. In that case, the compound is termed an aldose. Otherwise, the molecule has a ketone group, a carbonyl −(C=O)− between two carbons; then it is formally a ketone, and is termed a ketose. Ketoses of biological interest usually have the carbonyl at position 2.
The various classifications above can be combined, resulting in names such as "aldohexose" and "ketotriose".
A more general nomenclature for open-chain monosaccharides combines a Greek prefix to indicate the number of carbons (tri-, tetr-, pent-, hex-, etc.) with the suffixes "-ose" for aldoses and "-ulose" for ketoses. In the latter case, if the carbonyl is not at position 2, its position is then indicated by a numeric infix. So, for example, H(C=O)(CHOH)4H is pentose, H(CHOH)(C=O)(CHOH)3H is pentulose, and H(CHOH)2(C=O)(CHOH)2H is pent-3-ulose.
Open-chain stereoisomers
Two monosaccharides with equivalentmolecular graph
In chemical graph theory and in mathematical chemistry, a molecular graph or chemical graph is a representation of the structural formula of a chemical compound in terms of graph theory. A chemical graph is a labeled graph whose vertices corresp ...
s (same chain length and same carbonyl position) may still be distinct stereoisomer
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in ...
s, whose molecules differ in spatial orientation. This happens only if the molecule contains a stereogenic center, specifically a carbon atom that is chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
(connected to four distinct molecular sub-structures). Those four bonds can have any of two configurations in space distinguished by their handedness. In a simple open-chain monosaccharide, every carbon is chiral except the first and the last atoms of the chain, and (in ketoses) the carbon with the keto group.
For example, the triketose H(CHOH)(C=O)(CHOH)H (glycerone, dihydroxyacetone
Dihydroxyacetone (; DHA), also known as glycerone, is a simple saccharide (a triose) with formula .
DHA is primarily used as an ingredient in sunless tanning products. It is often derived from plant sources such as sugar beets and sugar cane, an ...
) has no stereogenic center, and therefore exists as a single stereoisomer. The other triose, the aldose H(C=O)(CHOH)2H (glyceraldehyde
Glyceraldehyde (glyceral) is a triose monosaccharide with chemical formula C3 H6 O3. It is the simplest of all common aldoses. It is a sweet, colorless, crystalline solid that is an intermediate compound in carbohydrate metabolism. The word comes ...
), has one chiral carbon—the central one, number 2—which is bonded to groups −H, −OH, −C(OH)H2, and −(C=O)H. Therefore, it exists as two stereoisomers whose molecules are mirror images of each other (like a left and a right glove). Monosaccharides with four or more carbons may contain multiple chiral carbons, so they typically have more than two stereoisomers. The number of distinct stereoisomers with the same diagram is bounded by 2''c'', where ''c'' is the total number of chiral carbons.
The Fischer projection
In chemistry, the Fischer projection, devised by Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates ...
is a systematic way of drawing the skeletal formula
The skeletal formula, or line-angle formula or shorthand formula, of an organic compound is a type of molecular structural formula that serves as a shorthand representation of a molecule's bonding and some details of its molecular geometry. A ...
of an acyclic monosaccharide so that the handedness of each chiral carbon is well specified. Each stereoisomer of a simple open-chain monosaccharide can be identified by the positions (right or left) in the Fischer diagram of the chiral hydroxyls (the hydroxyls attached to the chiral carbons).
Most stereoisomers are themselves chiral (distinct from their mirror images). In the Fischer projection, two mirror-image isomers differ by having the positions of all chiral hydroxyls reversed right-to-left. Mirror-image isomers are chemically identical in non-chiral environments, but usually have very different biochemical properties and occurrences in nature.
While most stereoisomers can be arranged in pairs of mirror-image forms, there are some non-chiral stereoisomers that are identical to their mirror images, in spite of having chiral centers. This happens whenever the molecular graph is symmetrical, as in the 3-ketopentoses H(CHOH)2(CO)(CHOH)2H, and the two halves are mirror images of each other. In that case, mirroring is equivalent to a half-turn rotation. For this reason, there are only three distinct 3-ketopentose stereoisomers, even though the molecule has two chiral carbons.
Distinct stereoisomers that are not mirror-images of each other usually have different chemical properties, even in non-chiral environments. Therefore, each mirror pair and each non-chiral stereoisomer may be given a specific monosaccharide name. For example, there are 16 distinct aldohexose stereoisomers, but the name "glucose" means a specific pair of mirror-image aldohexoses. In the Fischer projection, one of the two glucose isomers has the hydroxyl at left on C3, and at right on C4 and C5; while the other isomer has the reversed pattern. These specific monosaccharide names have conventional three-letter abbreviations, like "Glu" for glucose and "Thr" for threose
Threose is a four-carbon monosaccharide with molecular formula C4H8O4. It has a terminal aldehyde group rather than a ketone in its linear chain, and so is considered part of the aldose family of monosaccharides. The threose name can be used ...
.
Generally, a monosaccharide with ''n'' asymmetrical carbons has 2''n'' stereoisomers. The number of open chain stereoisomers for an aldose monosaccharide is larger by one than that of a ketose monosaccharide of the same length. Every ketose will have 2(''n''−3) stereoisomers where ''n'' > 2 is the number of carbons. Every aldose will have 2(''n''−2) stereoisomers where ''n'' > 2 is the number of carbons.
These are also referred to as epimers which have the different arrangement of −OH and −H groups at the asymmetric or chiral carbon atoms (this does not apply to those carbons having the carbonyl functional group).
Configuration of monosaccharides
Like many chiral molecules, the two stereoisomers of glyceraldehyde will gradually rotate the polarization direction of linearly polarized light as it passes through it, even in solution. The two stereoisomers are identified with the prefixes - and -, according to the sense of rotation: -glyceraldehyde isdextrorotatory
Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Circul ...
(rotates the polarization axis clockwise), while -glyceraldehyde is levorotatory
Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Circul ...
(rotates it counterclockwise).
Cyclisation of monosaccharides (hemiacetal formation)
A monosaccharide often switches from the acyclic (open-chain) form to acyclic
Cycle, cycles, or cyclic may refer to:
Anthropology and social sciences
* Cyclic history, a theory of history
* Cyclical theory, a theory of American political history associated with Arthur Schlesinger, Sr.
* Social cycle, various cycles in s ...
form, through a nucleophilic addition reaction between the carbonyl group and one of the hydroxyls of the same molecule. The reaction creates a ring of carbon atoms closed by one bridging oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
atom. The resulting molecule has a hemiacetal
A hemiacetal or a hemiketal has the general formula R1R2C(OH)OR, where R1 or R2 is hydrogen or an organic substituent. They generally result from the addition of an alcohol to an aldehyde or a ketone, although the latter are sometimes called hemi ...
or hemiketal
A hemiacetal or a hemiketal has the general formula R1R2C(OH)OR, where R1 or R2 is hydrogen or an organic substituent. They generally result from the addition of an alcohol to an aldehyde or a ketone, although the latter are sometimes called hemi ...
group, depending on whether the linear form was an aldose or a ketose. The reaction is easily reversed, yielding the original open-chain form.
In these cyclic forms, the ring usually has five or six atoms. These forms are called furanose
A furanose is a collective term for carbohydrates that have a chemical structure that includes a five-membered ring system consisting of four carbon atoms and one oxygen atom. The name derives from its similarity to the oxygen heterocycle furan, bu ...
s and pyranoses, respectively—by analogy with furan and pyran
In chemistry, pyran, or oxine, is a six-membered heterocyclic, non-aromatic ring, consisting of five carbon atoms and one oxygen atom and containing two double bonds. The molecular formula is C5H6O. There are two isomers of pyran that differ ...
, the simplest compounds with the same carbon-oxygen ring (although they lack the double bonds of these two molecules). For example, the aldohexose glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, u ...
may form a hemiacetal linkage between the aldehyde group on carbon 1 and the hydroxyl on carbon 4, yielding a molecule with a 5-membered ring, called glucofuranose. The same reaction can take place between carbons 1 and 5 to form a molecule with a ring, called glucopyranose. Cyclic forms with a seven-atom ring (the same of oxepane
Oxepane is a heterocyclic chemical compound with the formula C6H12O: a cycloheptane in which one methylene group is replaced by oxygen.
Oxepane can be polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or ma ...
), rarely encountered, are called heptose
A heptose is a monosaccharide with seven carbon atoms.
They have either an aldehyde functional group in position 1 (aldoheptoses) or a ketone functional group in position 2, 3 or 4 (ketoheptoses). Ketoheptoses have 4 chiral centers, whereas ald ...
s.


 For many monosaccharides (including glucose), the cyclic forms predominate, in the solid state and in solutions, and therefore the same name commonly is used for the open- and closed-chain isomers. Thus, for example, the term "glucose" may signify glucofuranose, glucopyranose, the open-chain form, or a mixture of the three.
Cyclization creates a new
For many monosaccharides (including glucose), the cyclic forms predominate, in the solid state and in solutions, and therefore the same name commonly is used for the open- and closed-chain isomers. Thus, for example, the term "glucose" may signify glucofuranose, glucopyranose, the open-chain form, or a mixture of the three.
Cyclization creates a new stereogenic
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups cr ...
center at the carbonyl-bearing carbon. The −OH group that replaces the carbonyl's oxygen may end up in two distinct positions relative to the ring's midplane. Thus each open-chain monosaccharide yields two cyclic isomers ( anomers), denoted by the prefixes α- and β-. The molecule can change between these two forms by a process called mutarotation, that consists in a reversal of the ring-forming reaction followed by another ring formation.
Haworth projection
The stereochemical structure of a cyclic monosaccharide can be represented in aHaworth projection
In chemistry, a Haworth projection is a common way of writing a structural formula to represent the cyclic structure of monosaccharides with a simple three-dimensional perspective. Haworth projection approximate the shapes of the actual mole ...
. In this diagram, the α-isomer for the pyranose form of a -aldohexose has the −OH of the anomeric carbon below the plane of the carbon atoms, while the β-isomer has the −OH of the anomeric carbon above the plane. Pyranoses typically adopt a chair conformation, similar to that of cyclohexane. In this conformation, the α-isomer has the −OH of the anomeric carbon in an axial position, whereas the β-isomer has the −OH of the anomeric carbon in equatorial position (considering -aldohexose sugars).
Derivatives
A large number of biologically important modified monosaccharides exist: * Amino sugars such as: **galactosamine
Galactosamine is a hexosamine derived from galactose with the molecular formula C6H13NO5. This amino sugar is a constituent of some glycoprotein hormones such as follicle-stimulating hormone (FSH) and luteinizing hormone (LH).
Precursors such ...
** glucosamine
Glucosamine (C6H13NO5) is an amino sugar and a prominent precursor in the biochemical synthesis of glycosylated proteins and lipids. Glucosamine is part of the structure of two polysaccharides, chitosan and chitin. Glucosamine is one of the most ...
** sialic acid
** ''N''-acetylglucosamine
* Sulfosugars such as:
** sulfoquinovose
* Others such as:
** ascorbic acid
Vitamin C (also known as ascorbic acid and ascorbate) is a water-soluble vitamin found in citrus and other fruits and vegetables, also sold as a dietary supplement and as a topical 'serum' ingredient to treat melasma (dark pigment spots) an ...
** mannitol
Mannitol is a type of sugar alcohol used as a sweetener and medication. It is used as a low calorie sweetener as it is poorly absorbed by the intestines. As a medication, it is used to decrease pressure in the eyes, as in glaucoma, and to lo ...
** glucuronic acid
See also
* Monosaccharide nomenclature *Reducing sugar
A reducing sugar is any sugar that is capable of acting as a reducing agent. In an alkaline solution, a reducing sugar forms some aldehyde or ketone, which allows it to act as a reducing agent, for example in Benedict's reagent. In such a reacti ...
* Sugar acid A Sugar acid or acidic sugar is a monosaccharide with a carboxyl group at one end or both ends of its chain.
Main classes of sugar acids include:
* Aldonic acids, in which the aldehyde group (−CHO) located at the initial end ( position 1) of an ...
* Sugar alcohol
Sugar alcohols (also called polyhydric alcohols, polyalcohols, alditols or glycitols) are organic compounds, typically derived from sugars, containing one hydroxyl group (–OH) attached to each carbon atom. They are white, water-soluble solids ...
Notes
References
* McMurry, John. Organic Chemistry. 7th ed. Belmont, CA: Thomson Brooks/Cole, 2008. Print.External links
Nomenclature of Carbohydrates
{{Authority control Carbohydrate chemistry