jelly roll fold on:
[Wikipedia]
[Google]
[Amazon]
 The jelly roll or Swiss roll fold is a
The jelly roll or Swiss roll fold is a
 A large number of viruses build their exterior capsids from proteins containing either a single or a double jelly roll fold. This shared capsid architecture is thought to reflect ancient evolutionary relationships, possibly dating to before the last universal common ancestor (LUCA) of cellular life. Other viral lineages use evolutionarily unrelated proteins to build their enclosed capsids, which likely evolved independently at least twice and possibly many times, with links to proteins of cellular origin.
A large number of viruses build their exterior capsids from proteins containing either a single or a double jelly roll fold. This shared capsid architecture is thought to reflect ancient evolutionary relationships, possibly dating to before the last universal common ancestor (LUCA) of cellular life. Other viral lineages use evolutionarily unrelated proteins to build their enclosed capsids, which likely evolved independently at least twice and possibly many times, with links to proteins of cellular origin.
 Double jelly roll capsid proteins consist of two single jelly roll folds connected by a short linker region. They are found in both
Double jelly roll capsid proteins consist of two single jelly roll folds connected by a short linker region. They are found in both
 A notable difference between PNGases F and the other double jelly roll proteins is the absence of the α-helices, which follow the F and F' strands in capsid proteins and DUF2961. The equivalent regions are variable in the PNGases F and contain either long loops or insertions. By contrast, jelly-roll domains of DUF2961 proteins contain an insertion of short β-hairpins upstream of the G and G' strands of the double jelly roll fold. Importantly, DUF2961 family proteins form trimers resembling viral capsomers.
A notable difference between PNGases F and the other double jelly roll proteins is the absence of the α-helices, which follow the F and F' strands in capsid proteins and DUF2961. The equivalent regions are variable in the PNGases F and contain either long loops or insertions. By contrast, jelly-roll domains of DUF2961 proteins contain an insertion of short β-hairpins upstream of the G and G' strands of the double jelly roll fold. Importantly, DUF2961 family proteins form trimers resembling viral capsomers.
Antiparallel β Domains
a section from ''Anatomy and Taxonomy of Protein Structure'' by Jane S. Richardson
by Jacqueline Humphries at ''Small Things Considered'', a blog sponsored by the
 The jelly roll or Swiss roll fold is a
The jelly roll or Swiss roll fold is a protein fold
A protein superfamily is the largest grouping (clade) of proteins for which common ancestry can be inferred (see homology). Usually this common ancestry is inferred from structural alignment and mechanistic similarity, even if no sequence simila ...
or supersecondary structure
A supersecondary structure is a compact three-dimensional protein structure of several adjacent elements of a secondary structure that is smaller than a protein domain or a subunit. Supersecondary structures can act as nucleations in the process ...
composed of eight beta strand
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a ge ...
s arranged in two four-stranded sheets. The name of the structure was introduced by Jane S. Richardson
Jane Shelby Richardson (born January 25, 1941) is an American biophysicist best known for developing the Richardson diagram, or ribbon diagram, a method of representing the 3D structure of proteins. Ribbon diagrams have become a standard repre ...
in 1981, reflecting its resemblance to the jelly or Swiss roll cake. The fold is an elaboration on the Greek key motif and is sometimes considered a form of beta barrel
In protein structures, a beta barrel is a beta sheet composed of tandem repeats that twists and coils to form a closed toroidal structure in which the first strand is bonded to the last strand (hydrogen bond). Beta-strands in many beta-barrels are ...
. It is very common in viral proteins, particularly viral capsid proteins. Taken together, the jelly roll and Greek key structures comprise around 30% of the all-beta proteins annotated in the Structural Classification of Proteins (SCOP) database.
Structure
The basic jelly roll structure consists of eightbeta strand
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a ge ...
s arranged in two four-stranded antiparallel beta sheets which pack together across a hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, t ...
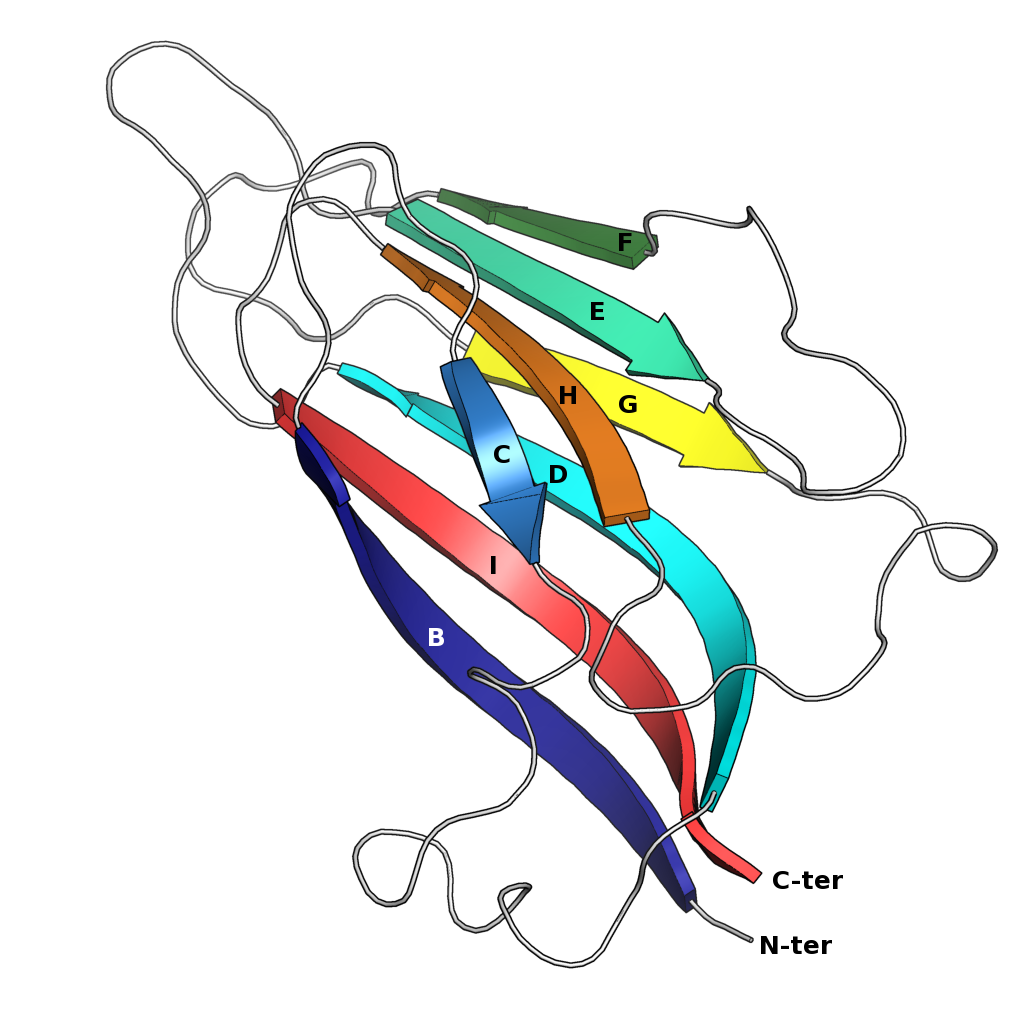
interface here citation... uniprot The strands are traditionally labeled B through I for the historical reason that the first solved structure, of a jelly roll capsid protein from the tomato bushy stunt virus
Tomato bushy stunt virus (TBSV) is a virus of the tombusvirus family. It was first reported in tomatoes in 1935 and primarily affects vegetable crops, though it is not generally considered an economically significant plant pathogen. Depending ...
, had an additional strand A outside the fold's common core. The sheets are composed of strands BIDG and CHEF, folded such that strand B packs opposite strand C, I opposite H, etc.
Viral proteins
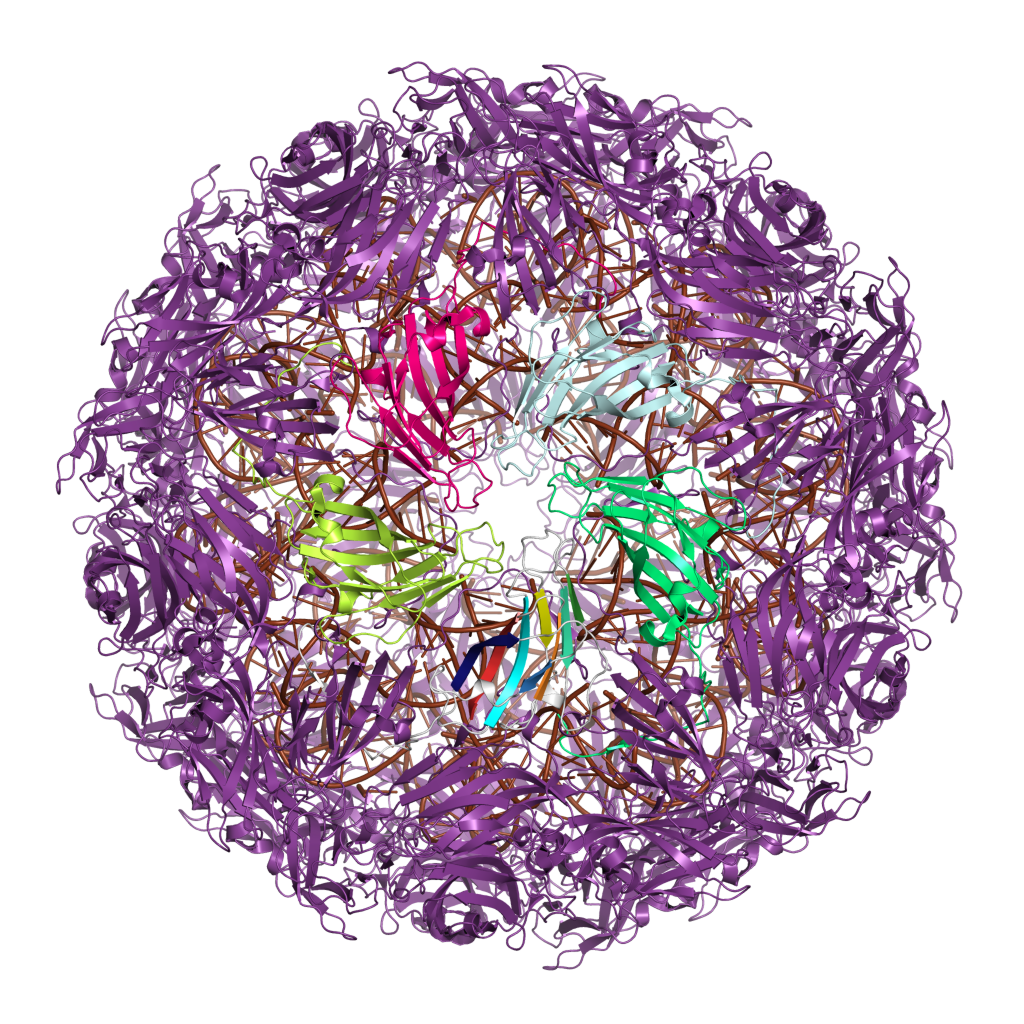
 A large number of viruses build their exterior capsids from proteins containing either a single or a double jelly roll fold. This shared capsid architecture is thought to reflect ancient evolutionary relationships, possibly dating to before the last universal common ancestor (LUCA) of cellular life. Other viral lineages use evolutionarily unrelated proteins to build their enclosed capsids, which likely evolved independently at least twice and possibly many times, with links to proteins of cellular origin.
A large number of viruses build their exterior capsids from proteins containing either a single or a double jelly roll fold. This shared capsid architecture is thought to reflect ancient evolutionary relationships, possibly dating to before the last universal common ancestor (LUCA) of cellular life. Other viral lineages use evolutionarily unrelated proteins to build their enclosed capsids, which likely evolved independently at least twice and possibly many times, with links to proteins of cellular origin.
Single jelly roll capsid proteins
Single jelly roll capsid (JRC) proteins are found in at least sixteen distinct viral families, mostly with icosahedral capsid structures and including both RNA viruses andDNA virus
A DNA virus is a virus that has a genome made of deoxyribonucleic acid (DNA) that is replicated by a DNA polymerase. They can be divided between those that have two strands of DNA in their genome, called double-stranded DNA (dsDNA) viruses, and ...
es. Many viruses with single jelly roll capsids are positive-sense single-stranded RNA virus
Positive-strand RNA viruses (+ssRNA viruses) are a group of related viruses that have positive-sense, single-stranded genomes made of ribonucleic acid. The positive-sense genome can act as messenger RNA (mRNA) and can be directly translated int ...
es. Two groups of double-stranded DNA virus
A DNA virus is a virus that has a genome made of deoxyribonucleic acid (DNA) that is replicated by a DNA polymerase. They can be divided between those that have two strands of DNA in their genome, called double-stranded DNA (dsDNA) viruses, and ...
es with single-JRC capsids are the ''Papillomaviridae
''Papillomaviridae'' is a family of non- enveloped DNA viruses whose members are known as papillomaviruses. Several hundred species of papillomaviruses, traditionally referred to as "types", have been identified infecting all carefully inspected ...
'' and ''Polyomaviridae
''Polyomaviridae'' is a family of viruses whose natural hosts are primarily mammals and birds. As of 2020, there are six recognized genera and 117 species, five of which are unassigned to a genus. 14 species are known to infect humans, while oth ...
'', both of which have fairly small capsids; in these viruses, the architecture of the assembled capsid orients the axis of the jelly roll parallel or "horizontally" relative to the capsid surface. A large-scale analysis of viral capsid components suggested that the single horizontal jelly roll is the most common fold among capsid proteins, accounting for about 28% of known examples.
Another group of viruses uses single jelly roll proteins in their capsids, but in the vertical rather than horizontal orientation. These viruses are evolutionarily related to the large group of double jelly-roll viruses known as the PRD1
''Tectiviridae'' is a family of viruses with 10 species in five genera. Bacteria serve as natural hosts. Tectiviruses have no head-tail structure, but are capable of producing tail-like tubes of ~ 60×10 nm upon adsorption or after chlorofo ...
-adenovirus
Adenoviruses (members of the family ''Adenoviridae'') are medium-sized (90–100 nm), nonenveloped (without an outer lipid bilayer) viruses with an icosahedral nucleocapsid containing a double-stranded DNA genome. Their name derives from thei ...
viral lineage, with similar capsid architecture realized through assembly of two distinct single jelly-roll major capsid proteins expressed from distinct genes. These single vertical jelly-roll viruses comprise the taxon Helvetiavirae
''Alphasphaerolipovirus'' is a genus
Genus ( plural genera ) is a taxonomic rank used in the biological classification of living and fossil organisms as well as viruses. In the hierarchy of biological classification, genus comes above s ...
. Known viruses with vertical single jelly roll capsids infect extremophilic
An extremophile (from Latin ' meaning "extreme" and Greek ' () meaning "love") is an organism that is able to live (or in some cases thrive) in extreme environments, i.e. environments that make survival challenging such as due to extreme temper ...
prokaryotes.
Double jelly roll proteins
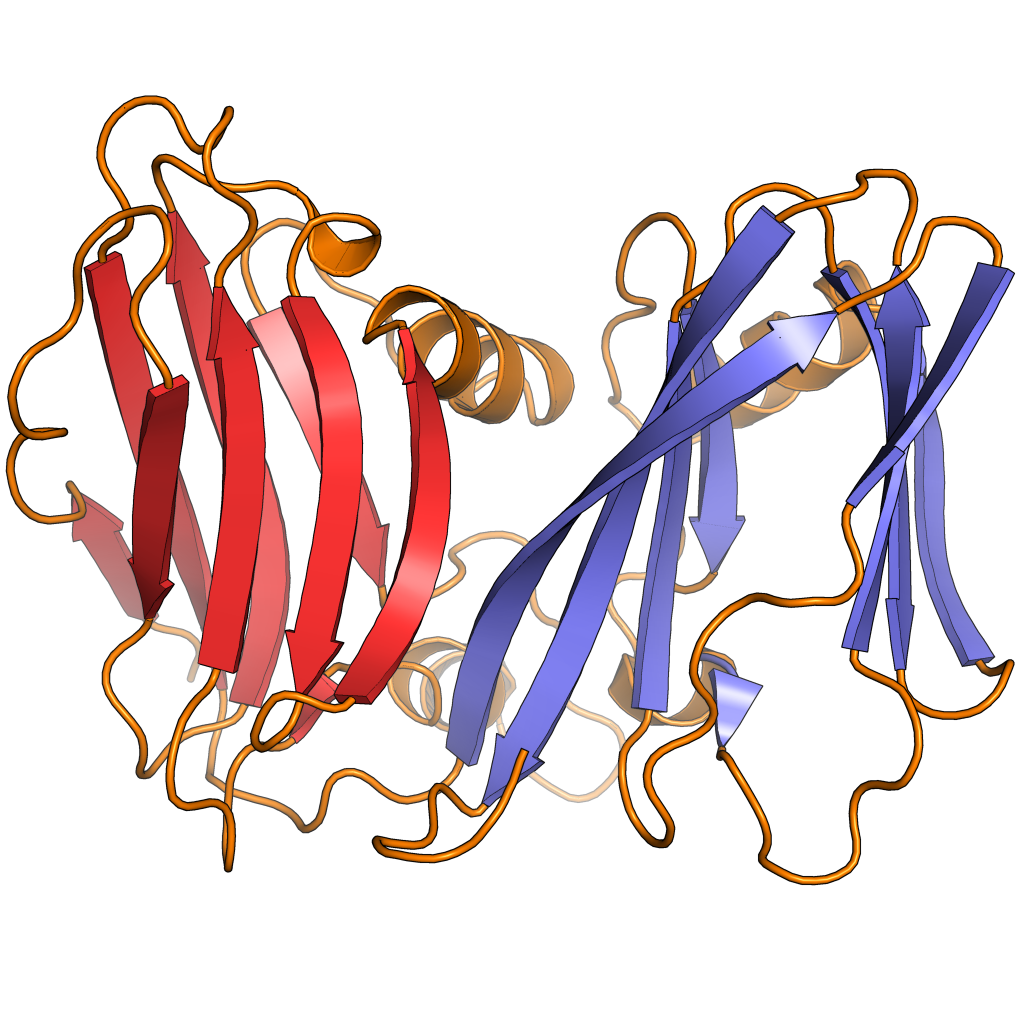
 Double jelly roll capsid proteins consist of two single jelly roll folds connected by a short linker region. They are found in both
Double jelly roll capsid proteins consist of two single jelly roll folds connected by a short linker region. They are found in both double-stranded DNA virus
A DNA virus is a virus that has a genome made of deoxyribonucleic acid (DNA) that is replicated by a DNA polymerase. They can be divided between those that have two strands of DNA in their genome, called double-stranded DNA (dsDNA) viruses, and ...
es and single-stranded DNA viruses
A DNA virus is a virus that has a genome made of deoxyribonucleic acid (DNA) that is replicated by a DNA polymerase. They can be divided between those that have two strands of DNA in their genome, called double-stranded DNA (dsDNA) viruses, and ...
of at least ten different viral families, including viruses that infect all domains of life, and spanning a large capsid size range. In the double jelly roll capsid architecture, the jelly roll axis is oriented perpendicular or "vertically" relative to the capsid surface.
Double jelly roll proteins are believed to have evolved from single jelly roll proteins by gene duplication
Gene duplication (or chromosomal duplication or gene amplification) is a major mechanism through which new genetic material is generated during molecular evolution. It can be defined as any duplication of a region of DNA that contains a gene. ...
. It is likely that vertical single jelly roll viruses represent a transitional form, and that the vertical and horizontal jelly roll capsid proteins have independent evolutionary origins from ancestral cellular proteins. The degree of structural similarity among double-jelly-roll virus capsids has led to the conclusion that these viruses likely have a common evolutionary origin despite their diversity in size and in host range; this has become known as the PRD1
''Tectiviridae'' is a family of viruses with 10 species in five genera. Bacteria serve as natural hosts. Tectiviruses have no head-tail structure, but are capable of producing tail-like tubes of ~ 60×10 nm upon adsorption or after chlorofo ...
-adenovirus
Adenoviruses (members of the family ''Adenoviridae'') are medium-sized (90–100 nm), nonenveloped (without an outer lipid bilayer) viruses with an icosahedral nucleocapsid containing a double-stranded DNA genome. Their name derives from thei ...
lineage ( Bamfordvirae). Many members of this group have been identified through metagenomics
Metagenomics is the study of genetic material recovered directly from environmental or clinical samples by a method called sequencing. The broad field may also be referred to as environmental genomics, ecogenomics, community genomics or microb ...
and in some cases have few to no other viral genes in common. Although most members of this group have icosahedral capsid geometry, a few families such as the '' Poxviridae'' and ''Ascoviridae
''Ascoviridae'' is a family of double strand DNA viruses that infect primarily invertebrates, mainly noctuids and spodoptera species; it contains two genera, ''Ascovirus'', which contains three species, and ''Toursvirus'' with a single species ' ...
'' have oval or brick-shaped mature virions; poxviruses such as ''Vaccinia
''Vaccinia virus'' (VACV or VV) is a large, complex, enveloped virus belonging to the poxvirus family. It has a linear, double-stranded DNA genome approximately 190 kbp in length, which encodes approximately 250 genes. The dimensions of t ...
'' undergo dramatic conformational changes mediated by highly derived double jelly roll proteins during maturation and likely derive from an icosahedral ancestor. Shared double-jelly-roll capsid proteins, along with other homologous proteins, have also been cited in support of the proposed order
Order, ORDER or Orders may refer to:
* Categorization, the process in which ideas and objects are recognized, differentiated, and understood
* Heterarchy, a system of organization wherein the elements have the potential to be ranked a number of ...
'' Megavirales'' containing the nucleocytoplasmic large DNA virus
''Nucleocytoviricota'' is a phylum of viruses. Members of the phylum are also known as the nucleocytoplasmic large DNA viruses (NCLDV), which serves as the basis of the name of the phylum with the suffix - for virus phylum. These viruses are refe ...
es (NCLDV).
Initially, it was believed that double jelly roll proteins are unique to viruses, because they were not observed in cellular proteins. However, in 2022, comparison of protein structures revealed several families of bona fide cellular proteins with the double jelly roll fold
Non-capsid proteins
Single jelly rolls also occur in non-capsid viral proteins, including minor components of the assembledvirion
A virus is a submicroscopic infectious agent that replicates only inside the living cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and archaea.
Since Dmitri Ivanovsky's ...
as well as non-virion proteins such as polyhedrin ''For the three dimensional shape, see Polyhedron''
Polyhedrins are a type of viral protein that form occlusion bodies (also called polyhedra), large structures that protect the virus particles from the outside environment for extended periods ...
.
Cellular proteins
Both single and double jelly roll folds are found in proteins of cellular origin. One class of cellular proteins with single jelly roll fold is thenucleoplasmin
Nucleoplasmin, the first identified molecular chaperone is a thermostable acidic protein with a pentameric structure. The protein was first isolated from Xenopus species
Functions
The pentameric protein participates in various significant cellul ...
s, which serve as molecular chaperone
In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of molecular chaperones, all of which function to ass ...
proteins for histone assembly into nucleosomes. The N-terminal domain of nucleoplasmins possesses a single jelly roll fold and assembled into a pentamer. Similar structures have since been reported in additional groups of chromatin
Chromatin is a complex of DNA and protein found in eukaryotic cells. The primary function is to package long DNA molecules into more compact, denser structures. This prevents the strands from becoming tangled and also plays important roles in ...
remodeling proteins. Jelly roll motifs with identical beta-sheet connectivity are also found in tumor necrosis factor
Tumor necrosis factor (TNF, cachexin, or cachectin; formerly known as tumor necrosis factor alpha or TNF-α) is an adipokine and a cytokine. TNF is a member of the TNF superfamily, which consists of various transmembrane proteins with a homolog ...
ligands and proteins from the bacterium '' Yersinia pseudotuberculosis'' that belong to a class of viral and bacterial proteins known as superantigen
Superantigens (SAgs) are a class of antigens that result in excessive activation of the immune system. Specifically it causes non-specific activation of T-cells resulting in polyclonal T cell activation and massive cytokine release. SAgs are ...
s.
More broadly, the members of the extremely diverse cupin superfamily
The cupin superfamily is a diverse superfamily of proteins named after its conserved barrel domain (''cupa'' being the Latin term for a small barrel). The superfamily includes a wide variety of enzymes as well as non-enzymatic seed storage ...
are also often described as jelly rolls; though the common core of the cupin domain structure contains only six beta strands, many cupins have eight. Examples include the non-heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consist ...
dioxygenase enzymes and JmjC-family histone demethylases.
Cellular proteins with the double jelly roll fold include glycoside hydrolases of the DUF2961 family, peptide:N-glycosidase F (PNGases F) and peptidylglycine alpha-amidating monooxygenase
Peptidyl-glycine alpha-amidating monooxygenase is an enzyme that catalyzes the conversion of glycine amides to amides and glyoxylate.
The enzyme is involved in the biosynthesis of many signaling peptides and some fatty acid amides.
In humans, t ...
.
 A notable difference between PNGases F and the other double jelly roll proteins is the absence of the α-helices, which follow the F and F' strands in capsid proteins and DUF2961. The equivalent regions are variable in the PNGases F and contain either long loops or insertions. By contrast, jelly-roll domains of DUF2961 proteins contain an insertion of short β-hairpins upstream of the G and G' strands of the double jelly roll fold. Importantly, DUF2961 family proteins form trimers resembling viral capsomers.
A notable difference between PNGases F and the other double jelly roll proteins is the absence of the α-helices, which follow the F and F' strands in capsid proteins and DUF2961. The equivalent regions are variable in the PNGases F and contain either long loops or insertions. By contrast, jelly-roll domains of DUF2961 proteins contain an insertion of short β-hairpins upstream of the G and G' strands of the double jelly roll fold. Importantly, DUF2961 family proteins form trimers resembling viral capsomers.
Evolution
Comparative studies of proteins classified as jelly roll and Greek key structures suggest that the Greek key proteins evolved significantly earlier than their more topologically complex jelly roll counterparts.Structural bioinformatics
Structural bioinformatics is the branch of bioinformatics that is related to the analysis and prediction of the three-dimensional structure of biological macromolecules such as proteins, RNA, and DNA. It deals with generalizations about macromol ...
studies comparing virus capsid jelly-roll proteins to other proteins of known structure indicates that the capsid proteins form a well-separated cluster, suggesting that they are subject to a distinctive set of evolutionary constraints. One of the most notable features of viral capsid jelly roll proteins is their ability to form oligomers in a repeated tiling pattern to produce a closed protein shell; the cellular proteins that are most similar in fold and topology are mostly also oligomers. It has been proposed that viral jelly-roll capsid proteins have evolved from cellular jelly-roll proteins, potentially on several independent occasions, at the earliest stages of cellular evolution.
History and nomenclature
The name "jelly roll" was first used for the structure composed of an elaboration on the Greek key motif byJane S. Richardson
Jane Shelby Richardson (born January 25, 1941) is an American biophysicist best known for developing the Richardson diagram, or ribbon diagram, a method of representing the 3D structure of proteins. Ribbon diagrams have become a standard repre ...
in 1981 and was intended to reflect the structure's resemblance to a jelly or Swiss roll cake. The structure has been given a variety of descriptive names, including a wedge, beta barrel, and beta roll. The edges of the two sheets do not meet to form regular hydrogen bonding patterns, and so it is often not considered to be a true beta barrel
In protein structures, a beta barrel is a beta sheet composed of tandem repeats that twists and coils to form a closed toroidal structure in which the first strand is bonded to the last strand (hydrogen bond). Beta-strands in many beta-barrels are ...
, though the term is in common use in describing viral capsid architecture. Cellular proteins containing jelly roll-like structures may be described as a cupin fold, a JmjC fold, or a double-stranded beta helix.
References
{{reflist, 30emExternal links
Antiparallel β Domains
a section from ''Anatomy and Taxonomy of Protein Structure'' by Jane S. Richardson
by Jacqueline Humphries at ''Small Things Considered'', a blog sponsored by the
American Society for Microbiology
The American Society for Microbiology (ASM), originally the Society of American Bacteriologists, is a professional organization for scientists who study viruses, bacteria, fungi, algae, and protozoa as well as other aspects of microbiology. It w ...
Protein folds