|
Cupin
The cupin superfamily is a diverse superfamily of proteins named after its conserved barrel domain (''cupa'' being the Latin term for a small barrel). The superfamily includes a wide variety of enzymes as well as non-enzymatic seed storage proteins. Members of the superfamily play a role in allergy, especially seed storage proteins like 7S and 11S globulins, also known as vicilins and legumins, respectively. These proteins can be found at high concentrations in seeds of both mono- and dicotyledonous plants and are an important component of the normal human diet. History Thomas Burr Osborne at the end of the 19th century was the first person to systematically study seed storage proteins by their solubility characteristics. He established 4 classes of proteins: water-soluble albumins; salt soluble globulins: vicilin—typically having sedimentation coefficients, S values (a measure of the protein mass determined by sedimentation equilibrium ultracentrifugation) of about 7 S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
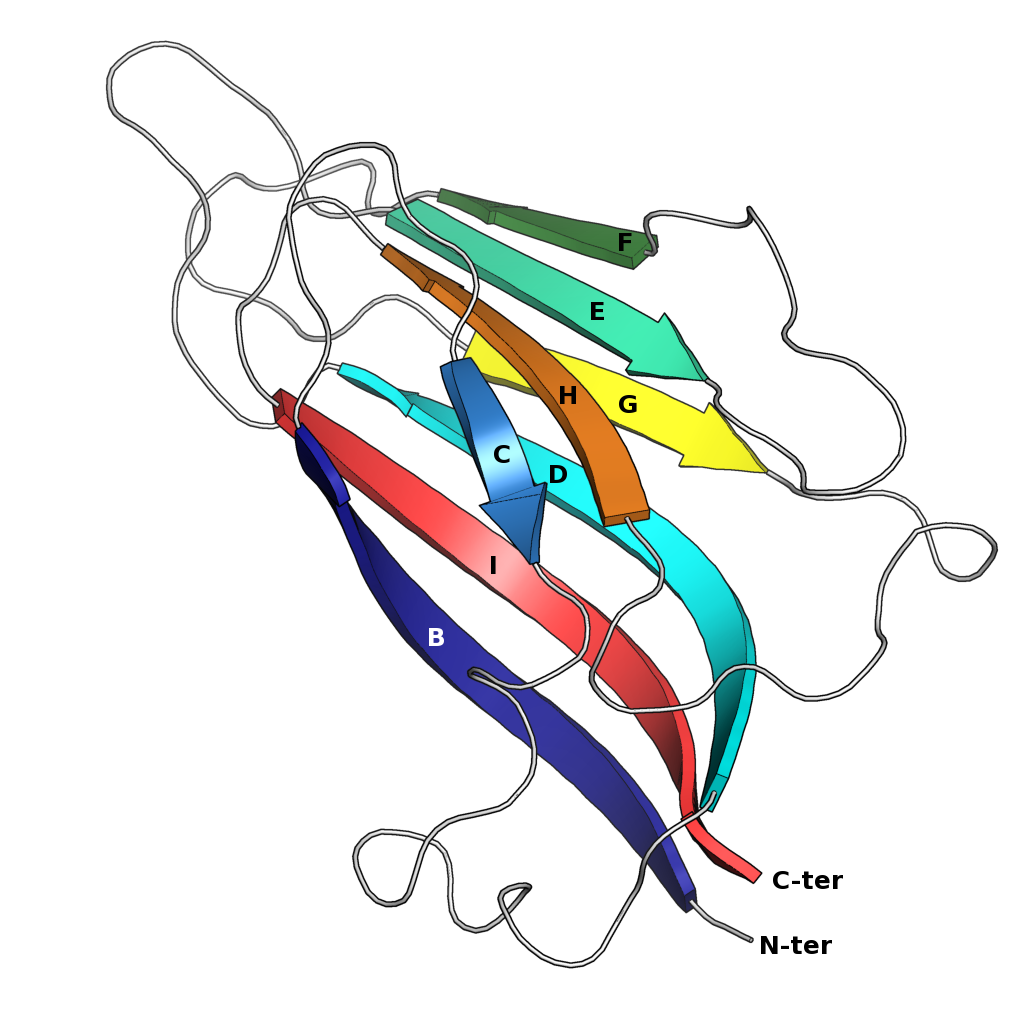
Jelly Roll Fold
The jelly roll or Swiss roll fold is a protein fold or supersecondary structure composed of eight beta strands arranged in two four-stranded sheets. The name of the structure was introduced by Jane S. Richardson in 1981, reflecting its resemblance to the jelly or Swiss roll cake. The fold is an elaboration on the Greek key motif and is sometimes considered a form of beta barrel. It is very common in viral proteins, particularly viral capsid proteins. Taken together, the jelly roll and Greek key structures comprise around 30% of the all-beta proteins annotated in the Structural Classification of Proteins (SCOP) database. Structure The basic jelly roll structure consists of eight beta strands arranged in two four-stranded antiparallel beta sheets which pack together across a hydrophobic interface here citation... uniprot The strands are traditionally labeled B through I for the historical reason that the first solved structure, of a jelly roll capsid protein from the tomato bu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vicilin
Vicilin is a legumin-associated globulin protein. Vicilin can be described as a storage protein found in legumes such as the pea or lentil. Vicilin is a protein that protects plants from fungi and microorganism. It has been hypothesized it's an allergen in pea allergy responses. Function of Vicilin Vicilin is a globulin present in legumes that assists the storage of proteins. Vicilins are 7S globulins. Sucrose binding, antifungal capabilities, and oxidative stress are a few of the globulin's functions. Vicilin peptides produced by digestion using trypsin or chymotrypsin offer anti-hypersensitive properties. Vicilin's function was best understood because to the addition of the copper ligand. Vicilin has various significant residues, four of which are involved in copper ion coordination. Vicilin belongs to the cupin family of proteins, therefore metal ligand coordination is common, but Vicilin is the only seed storage protein in this family known to have copper. Due to various e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacillus Subtilis
''Bacillus subtilis'', known also as the hay bacillus or grass bacillus, is a Gram-positive, catalase-positive bacterium, found in soil and the gastrointestinal tract of ruminants, humans and marine sponges. As a member of the genus ''Bacillus'', ''B. subtilis'' is rod-shaped, and can form a tough, protective endospore, allowing it to tolerate extreme environmental conditions. ''B. subtilis'' has historically been classified as an obligate aerobe, though evidence exists that it is a facultative anaerobe. ''B. subtilis'' is considered the best studied Gram-positive bacterium and a model organism to study bacterial chromosome replication and cell differentiation. It is one of the bacterial champions in secreted enzyme production and used on an industrial scale by biotechnology companies. Description ''Bacillus subtilis'' is a Gram-positive bacterium, rod-shaped and catalase-positive. It was originally named ''Vibrio subtilis'' by Christian Gottfried Ehrenberg, and renamed ''B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shewanella Oneidensis
''Shewanella oneidensis'' is a bacterium notable for its ability to reduce metal ions and live in environments with or without oxygen. This proteobacterium was first isolated from Lake Oneida, NY in 1988, hence its name. ''S. oneidensis'' is a facultative bacterium, capable of surviving and proliferating in both aerobic and anaerobic conditions. The special interest in ''S. oneidensis'' MR-1 revolves around its behavior in an anaerobic environment contaminated by heavy metals such as iron, lead and uranium. Experiments suggest it may reduce ionic mercury to elemental mercury and ionic silver to elemental silver. Cellular respiration for these bacteria is not restricted to heavy metals though; the bacteria can also target sulfates, nitrates and chromates when grown anaerobically. Name This species is referred to as ''S. oneidensis'' MR-1, indicating "manganese reducing", a special feature of this organism. It is a common misconception to think that MR-1 refers to "metal-reduc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Superfamily
A protein superfamily is the largest grouping (clade) of proteins for which common ancestry can be inferred (see homology (biology), homology). Usually this common ancestry is inferred from structural alignment and mechanistic similarity, even if no sequence similarity is evident. Sequence homology can then be deduced even if not apparent (due to low sequence similarity). Superfamilies typically contain several protein families which show sequence similarity within each family. The term ''protein clan'' is commonly used for protease and glycosyl hydrolases superfamilies based on the MEROPS and CAZy classification systems. Identification Superfamilies of proteins are identified using a number of methods. Closely related members can be identified by different methods to those needed to group the most evolutionarily divergent members. Sequence similarity Historically, the similarity of different amino acid sequences has been the most common method of inferring Sequence homology, h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Barrel
In protein structures, a beta barrel is a beta sheet composed of tandem repeats that twists and coils to form a closed toroidal structure in which the first strand is bonded to the last strand (hydrogen bond). Beta-strands in many beta-barrels are arranged in an antiparallel fashion. Beta barrel structures are named for resemblance to the barrels used to contain liquids. Most of them are water-soluble proteins and frequently bind hydrophobic ligands in the barrel center, as in lipocalins. Others span cell membranes and are commonly found in porins. Porin-like barrel structures are encoded by as many as 2–3% of the genes in Gram-negative bacteria. It has been shown that more than 600 proteins with various function (e.g., oxidase, dismutase, amylase) contain the beta barrel structure. In many cases, the strands contain alternating polar and non-polar (hydrophilic and hydrophobic) amino acids, so that the hydrophobic residues are oriented into the interior of the barrel to form a hy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzymes
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are Ribozyme, catalytic RNA molecules, called ribozymes. Enzymes' Chemical specificity, specific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Storage Protein
Storage proteins serve as biological reserves of metal ions and amino acids, used by organisms. They are found in plant seeds, egg whites, and milk. Ferritin is an example of a storage protein that stores iron. Iron is a component of heme, which is contained in the transport protein, hemoglobin and in cytochromes. Some storage proteins store amino acids. Storage proteins' amino acids are used in embryonic development of animals or plants. Two amino acid storage proteins in animals are casein and ovalbumin. Seeds, particularly of leguminous plants, contain high concentrations of storage proteins. Up to 25 percent of the dry weight of the seed can be composed of storage proteins. The best known storage protein in wheat is the prolamin gliadin, a component of gluten Gluten is a structural protein naturally found in certain cereal grains. Although "gluten" often only refers to wheat proteins, in medical literature it refers to the combination of prolamin and glutelin protein ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Legumin
Legumin is family of globular proteins obtained from beans, peas, lentils, vetches, hemp (specifically edestin) and other leguminous seeds. Edestin is a biologically active legumin protein that is digestible for human bodies. Garden peas are a common nutritional source for humans that contains legumin. Legumin is similar to the casein of mammalian milk and was called "vegetable casein" since it was considered analogous to the mammalian protein. The primary function of the legumin protein in seeds is storage. Legumin proteins are one of the main storage proteins of angiosperms and gymnosperms. Legumin is an insoluble hexameric conjugated protein with a high concentration of carbon and oxygen. Properties Structure Legumin is a conjugated protein with six subunits. The individual subunits have a hydrophilic α chain that is initially linked to the smaller hydrophobic β chain with a peptide bond. Both the α and β chains are encoded by the same gene. Each of the six subunits ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thomas Burr Osborne (chemist)
Thomas Burr Osborne (August 5, 1859 – January 29, 1929) was a biochemist who, with Lafayette Mendel, independently discovered Vitamin A, though Elmer McCollum and Marguerite Davis were ultimately given credit, as they had submitted their paper first by three weeks. He is known for his work isolating and characterizing seed proteins, and for determining protein nutritional requirements. His career was spent at the Connecticut Agricultural Experiment Station. Biography Thomas Burr Osborne was born in New Haven, Connecticut on August 5, 1859. He was the son of lawyer Arthur Dimon Osborne and the grandson of US Representative Thomas Burr Osborne. He earned an undergraduate degree from Yale College in 1881, and a PhD in chemistry there in 1885. He married Elizabeth Annah Johnson on June 23, 1886, and they had one son. Osborne died at his home in New Haven on January 29, 1929. Career His life exhibited "a single purpose, the understanding of the relationships of proteins to eac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lafayette Mendel
Lafayette Benedict Mendel (February 5, 1872 – December 9, 1935) was an American biochemist known for his work in nutrition, with longtime collaborator Thomas B. Osborne, including the study of Vitamin A, Vitamin B, lysine and tryptophan. Life Mendel was born in Delhi, New York, son of Benedict Mendel, a merchant born in Aufhausen, Germany in 1833, and Pauline Ullman, born in Eschenau, Germany. His father immigrated to the United States from Germany in 1851, his mother in 1870."Lafayette Benedict Mendel." World of Biology. Farmington Hills, Mich.: Thomson Gale. 2006. At 15, he won a New York State scholarship. Mendel studied classics, economics and the humanities, as well as biology and chemistry at |