glyoxylate fermentation on:
[Wikipedia]
[Google]
[Amazon]
Glyoxylic acid or oxoacetic acid is an organic compound. Together with
 In solution, the monohydrate exists in equilibrium with a hemi acylal dimer form:Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim.
:
In solution, the monohydrate exists in equilibrium with a hemi acylal dimer form:Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim.
: In isolation, the aldehyde structure has as a major conformer a cyclic
In isolation, the aldehyde structure has as a major conformer a cyclic 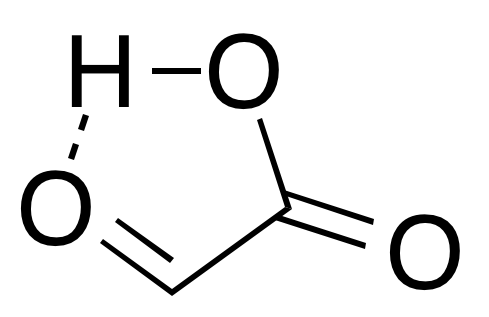 The Henry's law constant of glyoxylic acid is KH = 1.09 × 104 × exp 40.0 × 103/R) × (1/T − 1/298)
The Henry's law constant of glyoxylic acid is KH = 1.09 × 104 × exp 40.0 × 103/R) × (1/T − 1/298)


acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component ...
, glycolic acid, and oxalic acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and formula . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early inve ...
, glyoxylic acid is one of the C2 carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
s. It is a colourless solid that occurs naturally and is useful industrially.
Structure and nomenclature
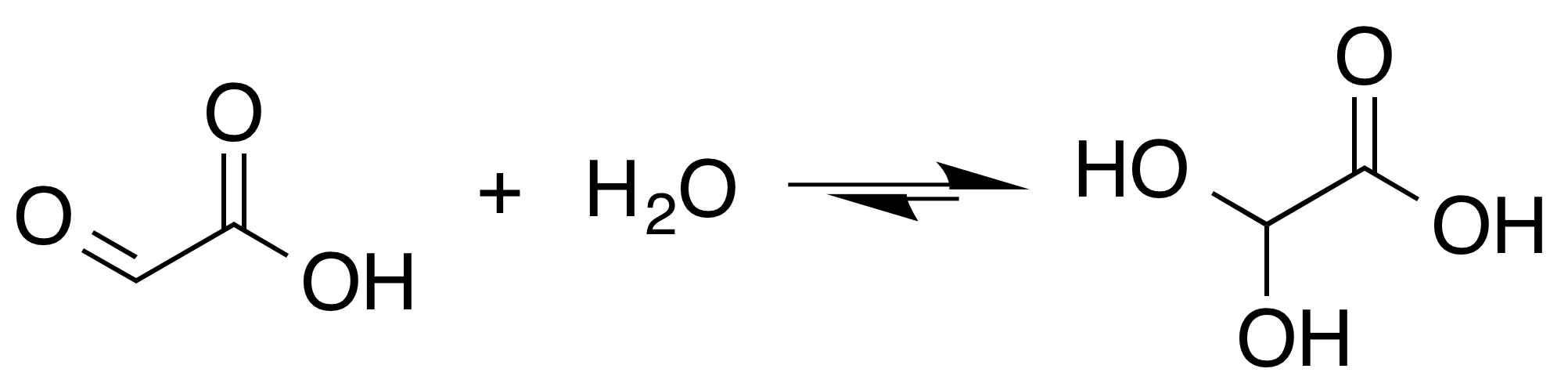
Although the structure of glyoxylic acid is described as having an aldehyde functional group, the aldehyde is only a minor component of the form most prevalent in some situations. Instead, it often exists as a hydrate or a cyclicdimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* Julius Dimer ( ...
. For example, in the presence of water, the carbonyl rapidly converts to a geminal diol (described as the "monohydrate"). The equilibrium constant (''K'') is 300 for the formation of dihydroxyacetic acid at room temperature:
: In solution, the monohydrate exists in equilibrium with a hemi acylal dimer form:Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim.
:
In solution, the monohydrate exists in equilibrium with a hemi acylal dimer form:Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim.
: In isolation, the aldehyde structure has as a major conformer a cyclic
In isolation, the aldehyde structure has as a major conformer a cyclic hydrogen-bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing ...
ed structure with the aldehyde carbonyl in close proximity to the carboxyl hydrogen:
: The Henry's law constant of glyoxylic acid is KH = 1.09 × 104 × exp 40.0 × 103/R) × (1/T − 1/298)
The Henry's law constant of glyoxylic acid is KH = 1.09 × 104 × exp 40.0 × 103/R) × (1/T − 1/298)
Preparations
The conjugate base of glyoxylic acid is known as glyoxylate and is the form that the compound exists in solution at neutral pH. Glyoxylate is the byproduct of the amidation process in biosynthesis of several amidated peptides. For the historical record, glyoxylic acid was prepared from oxalic acid electrosynthetically: in organic synthesis, lead dioxide cathodes were applied for preparing glyoxylic acid fromoxalic acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and formula . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early inve ...
in a sulfuric acid electrolyte.
:380px
38 may refer to:
*38 (number), the natural number following 37 and preceding 39
*one of the years 38 BC, AD 38, 1938, 2038
*.38, a caliber of firearms and cartridges
**.38 Special, a revolver cartridge
*'' Thirty-Eight: The Hurricane That Transfo ...
Hot nitric acid can oxidize glyoxal to glyoxylic; however this reaction is highly exothermic and prone to thermal runaway. In addition, oxalic acid is the main side product.
Also, ozonolysis of maleic acid is effective.
Biological role
Glyoxylate is an intermediate of the glyoxylate cycle, which enables organisms, such as bacteria, fungi, and plants to convert fatty acids into carbohydrates. The glyoxylate cycle is also important for induction of plant defense mechanisms in response to fungi. The glyoxylate cycle is initiated through the activity of isocitrate lyase, which converts isocitrate into glyoxylate and succinate. Research is being done to co-opt the pathway for a variety of uses such as the biosynthesis of succinate.In humans
Glyoxylate is produced via two pathways: through the oxidation of glycolate in peroxisomes or through the catabolism of hydroxyproline in mitochondria. In the peroxisomes, glyoxylate is converted into glycine by AGT1 or into oxalate by glycolate oxidase. In the mitochondria, glyoxylate is converted into glycine by AGT2 or into glycolate by glyoxylate reductase. A small amount of glyoxylate is converted into oxalate by cytoplasmic lactate dehydrogenase.
In plants
In addition to being an intermediate in the glyoxylate cycle, glyoxylate is also an important intermediate in thephotorespiration
Photorespiration (also known as the oxidative photosynthetic carbon cycle or C2 cycle) refers to a process in plant metabolism where the enzyme RuBisCO oxygenates RuBP, wasting some of the energy produced by photosynthesis. The desired reaction i ...
pathway. Photorespiration is a result of the side reaction of RuBisCO with O2 instead of CO2. While at first considered a waste of energy and resources, photorespiration has been shown to be an important method of regenerating carbon and CO2, removing toxic phosphoglycolate, and initiating defense mechanisms. In photorespiration, glyoxylate is converted from glycolate through the activity of glycolate oxidase in the peroxisome. It is then converted into glycine through parallel actions by SGAT and GGAT, which is then transported into the mitochondria. It has also been reported that the pyruvate dehydrogenase complex may play a role in glycolate and glyoxylate metabolism.

Disease relevance
Diabetes
Glyoxylate is thought to be a potential early marker for Type II diabetes. One of the key conditions of diabetes pathology is the production of advanced glycation end-products (AGEs) caused by thehyperglycemia
Hyperglycemia is a condition in which an excessive amount of glucose circulates in the blood plasma. This is generally a blood sugar level higher than 11.1 mmol/L (200 mg/dL), but symptoms may not start to become noticeable until even ...
. AGEs can lead to further complications of diabetes, such as tissue damage and cardiovascular disease. They are generally formed from reactive aldehydes, such as those present on reducing sugars and alpha-oxoaldehydes. In a study, glyoxylate levels were found to be significantly increased in patients who were later diagnosed with Type II diabetes. The elevated levels were found sometimes up to three years before the diagnosis, demonstrating the potential role for glyoxylate to be an early predictive marker.
Nephrolithiasis
Glyoxylate is involved in the development of hyperoxaluria, a key cause of nephrolithiasis (commonly known as kidney stones). Glyoxylate is both a substrate and inductor of sulfate anion transporter-1 (sat-1), a gene responsible for oxalate transportation, allowing it to increase sat-1 mRNA expression and as a result oxalate efflux from the cell. The increased oxalate release allows the buildup of calcium oxalate in the urine, and thus the eventual formation of kidney stones. The disruption of glyoxylate metabolism provides an additional mechanism of hyperoxaluria development. Loss of function mutations in theHOGA1
4-Hydroxy-2-oxoglutarate aldolase, mitochondrial (HOGA1) also known as dihydrodipicolinate synthase-like (DHDPSL) is an enzyme that in humans is encoded by the HOGA1 gene. The protein is one of the enzymes (4-hydroxy-2-oxoglutarate aldolase) invol ...
gene leads to a loss of the 4-hydroxy-2-oxoglutarate aldolase, an enzyme in the hydroxyproline to glyoxylate pathway. The glyoxylate resulting from this pathway is normally stored away to prevent oxidation to oxalate in the cytosol. The disrupted pathway, however, causes a buildup of 4-hydroxy-2-oxoglutarate which can also be transported to the cytosol and converted into glyoxylate through a different aldolase. These glyoxylate molecules can be oxidized into oxalate increasing its concentration and causing hyperoxaluria.
Reactions and uses
Glyoxylic acid is about ten times stronger an acid thanacetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component ...
, with an acid dissociation constant
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction
:HA ...
of 4.7 × 10−4 (p''K''a = 3.32):
:OCHCO2H + H+
With base, glyoxylic acid disproportionates
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. More generally, the term can ...
, forming hydroxyacetic acid
Glycolic acid (or hydroxyacetic acid; chemical formula HOCH2CO2H) is a colorless, odorless and hygroscopic crystalline solid, highly soluble in water. It is used in various skin-care products. Glycolic acid is widespread in nature. A glycolate (so ...
and oxalic acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and formula . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early inve ...
:
:2 OCHCO2H + H2O → HOCH2CO2H + HO2CCO2H
Glyoxylic acid gives heterocycles upon condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor to ...
with urea and 1,2-diaminobenzene
''o''-Phenylenediamine (OPD) is an organic compound with the formula C6H4(NH2)2. This aromatic diamine is an important precursor to many heterocyclic compounds. It is isomeric with ''m''-phenylenediamine and ''p''-phenylenediamine.
Preparation ...
.
Phenol derivatives
In general, glyoxylic acid undergoes an electrophilic aromatic substitution reaction with phenols, a versatile step in the synthesis of several other compounds. The immediate product with phenol itself is4-hydroxymandelic acid
4-Hydroxymandelic acid is a chemical compound used to synthesize atenolol. The compound typically occurs as a monohydrate.
Synthesis and occurrence
It is produced from 4-hydroxypyruvic acid by the action of the enzyme (''S'')-''p''-hydroxymand ...
. This species reacts with ammonia to give hydroxyphenylglycine, a precursor to the drug amoxicillin. Reduction of the 4-hydroxymandelic acid gives 4-hydroxyphenylacetic acid
4-Hydroxyphenylacetic acid is a chemical compound found in olive oil and beer.
Synthesis
4-Hydroxyphenylacetic acid is obtained by reducing 4-Hydroxymandelic acid, 4-hydroxymandelic acid with elemental phosphorus and iodine.
Uses
In industry, ...
, a precursor to the drug atenolol.
The sequence of reactions, in which glyoxylic acid reacts with guaiacol the phenolic component followed by oxidation and decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is t ...
, provides a route to vanillin as a net formylation
In biochemistry, the addition of a formyl functional group is termed formylation. A formyl functional group consists of a carbonyl bonded to hydrogen. When attached to an R group, a formyl group is called an aldehyde.
Formylation has been identi ...
process.
Hopkins Cole reaction
Glyoxylic acid is a component of theHopkins–Cole reaction The Hopkins-Cole reaction, also known as the glyoxylic acid reaction, is a chemical test used for detecting the presence of tryptophan in proteins. A protein solution is mixed with Hopkins Cole reagent, which consists of glyoxylic acid. Concentrated ...
, used to check for the presence of tryptophan in proteins.
Environmental chemistry
Glyoxylic acid is one of several ketone- and aldehyde-containing carboxylic acids that together are abundant insecondary organic aerosol A secondary organic aerosol (SOA) is a molecule produced via oxidation over several generations of a parent organic molecule. In contrast to primary organic aerosols, which are emitted directly from the biosphere, secondary organic aerosols are eith ...
s. In the presence of water and sunlight, glyoxylic acid can undergo photochemical oxidation. Several different reaction pathways can ensue, leading to various other carboxylic acid and aldehyde products.
Safety
The compound is not very toxic with an for rats of 2500 mg/kg.References