|
Formylation
Formylation refers to any chemical processes in which a compound is functionalized with a formyl group (-CH=O). In organic chemistry, the term is most commonly used with regards to aromatic compounds (for example the conversion of benzene to benzaldehyde in the Gattermann–Koch reaction). In biochemistry the reaction is catalysed by enzymes such as formyltransferases. Formylation generally involves the use of formylation agents, reagents that give rise to the CHO group. Among the many formylation reagents, particularly important are formic acid and carbon monoxide. A formylation reaction in organic chemistry refers to organic reactions in which an organic compound is functionalized with a formyl group (-CH=O). The reaction is a route to aldehydes (''C''-CH=O), formamides (''N''-CH=O), and formate#Formate esters, formate esters (''O''-CH=O). Formylation agents A reagent that delivers the formyl group is called a formylating agent. * Formic acid * Dimethylformamide and phosphorus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Duff Reaction
The Duff reaction or hexamine aromatic formylation is a formylation reaction used in organic chemistry for the synthesis of benzaldehydes with hexamine as the formyl carbon source. The method is generally inefficient. The reaction is named after James Cooper Duff. The reaction requires strongly polar effect, electron donating substituents on the aromatic ring such as in a phenol. Formylation occurs ''arene substitution patterns, ortho'' to the electron donating substituent preferentially, unless the ''ortho'' positions are blocked, in which case the formylation occurs at the ''para'' position. Examples The modified salicylaldehyde 3,5-di-tert-butylsalicylaldehyde, 3,5-di-''tert''-butylsalicylaldehyde is prepared by the Duff reaction: The natural product syringaldehyde can also be prepared by the Duff reaction. In this example, formylation occurs at the position para to the phenolic OH. Unlike other formylation reactions the Duff reaction is able to attach multiple aldehy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gattermann Reaction
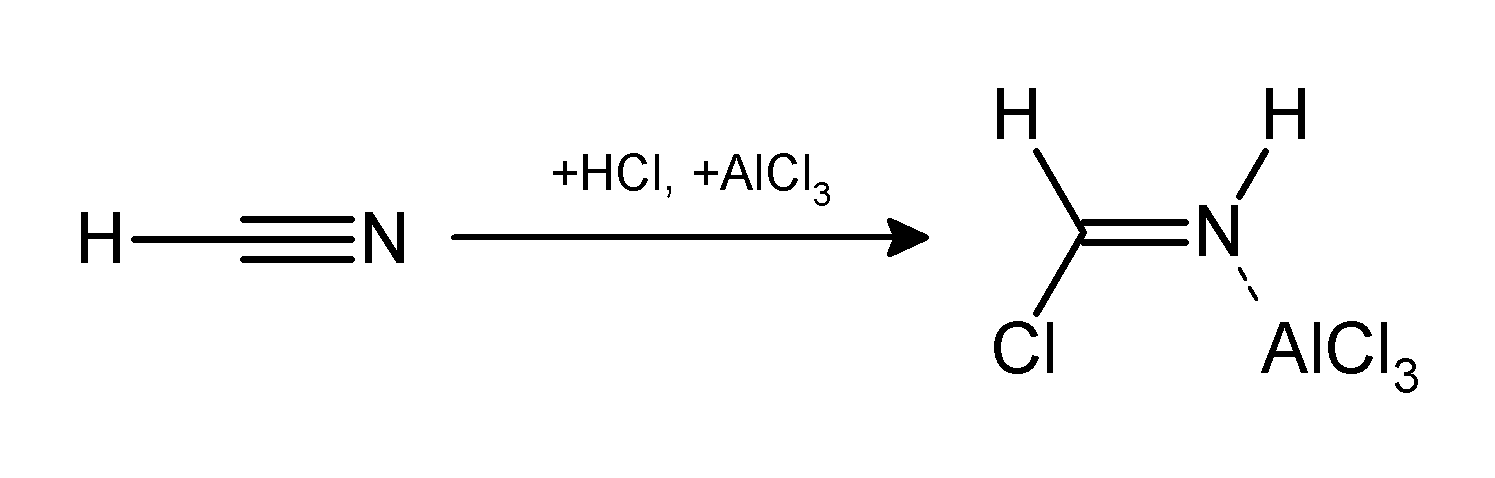
The Gattermann reaction (also known as the Gattermann formylation and the Gattermann salicylaldehyde synthesis) is a chemical reaction in which aromatic compounds are formylated by a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) in the presence of a Lewis acid catalyst such as aluminium chloride (AlCl3). It is named for the German chemist Ludwig Gattermann and is similar to the Friedel–Crafts reaction. Modifications have shown that it is possible to use sodium cyanide or cyanogen bromide in place of hydrogen cyanide. The reaction can be simplified by replacing the HCN/AlCl3 combination with zinc cyanide. Although it is also highly toxic, Zn(CN)2 is a solid, making it safer to work with than gaseous HCN. The Zn(CN)2 reacts with the HCl to form the key HCN reactant and Zn(Cl)2 that serves as the Lewis-acid catalyst ''in-situ''. An example of the Zn(CN)2 method is the synthesis of mesitaldehyde from mesitylene. Gattermann–Koch reaction The Gattermann– ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology. Structure and bonding Aldehyde molecules have a central carbon atom that is connected by a double bond to oxygen, a single bond to hydrogen and another single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The bond length is about 120–122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes such as formaldehyde and acetaldehyde are solubl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Oxychloride
Phosphoryl chloride (commonly called phosphorus oxychloride) is a colourless liquid with the formula . It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters. Structure Like phosphate, is tetrahedral in shape. It features three P−Cl bonds and one strong P–O bond, with an estimated bond dissociation energy of 533.5 kJ/mol. Unlike in the case of , the Schomaker-Stevenson rule predicts appropriate bond length for the P–O bond only if the P–O bond is treated as a double bond, P=O. More modern treatments explain the tight P–O bond as a combination of lone pair transfer from the phosphorus to the oxygen atom and a dative ''π'' back-bond that produces an effective + −configuration. Phosphoryl chloride exists as neutral molecules in the solid, liquid and gas states. This is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Cyanide
Zinc cyanide is the inorganic compound with the formula Zn( CN)2. It is a white solid that is used mainly for electroplating zinc but also has more specialized applications for the synthesis of organic compounds. Structure In Zn(CN)2, zinc adopts the tetrahedral coordination environment, all linked by bridging cyanide ligands. The structure consists of two "interpenetrating" structures (blue and red in the picture above). Such motifs are sometimes called "expanded diamondoid" structures. Some forms of SiO2 adopt a similar structure, wherein the tetrahedral Si centres are linked by oxides. The cyanide group shows head to tail disorder with any zinc atom having between one and four carbon neighbours, and the remaining being nitrogen atoms. It shows one of the largest negative coefficients of thermal expansion (exceeding the previous record holder, zirconium tungstate). Chemical properties Typical for an inorganic polymer, Zn(CN)2 is insoluble in most solvents. The solid diss ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Cyanide
Hydrogen cyanide (formerly known as prussic acid) is a chemical compound with the chemical formula, formula HCN and structural formula . It is a highly toxic and flammable liquid that boiling, boils slightly above room temperature, at . HCN is produced on an industrial scale and is a highly valued Precursor (chemistry), precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its Volatility (chemistry), volatile nature. A solution of hydrogen cyanide in water (molecule), water, represented as HCN(aqueous, aq), is called ''hydrocyanic acid''. The Salt (chemistry), salts of the cyanide anion are known as cyanides. Whether hydrogen cyanide is an organic compound or not is a topic of debate among chemists, and opinions vary from author to author. Traditionally, it is considered ino ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Chloride
The Chemical compound, compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride gas and hydrochloric acid are important in technology and industry. Hydrochloric acid, the aqueous solution of hydrogen chloride, is also commonly given the formula HCl. Reactions Hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom H and a chlorine atom Cl connected by a Polar-covalent bond, polar covalent bond. The chlorine atom is much more Electronegativity, electronegative than the hydrogen atom, which makes this bond polar. Consequently, the molecule has a large Molecular dipole moment, dipole moment with a negative partial charge (δ−) at the chlorine atom and a positive partial charge (δ+) at the hydrogen atom. In part because of its high polarity, HCl is very soluble in water (and in other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanide
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom. Ionic cyanides contain the cyanide anion . This anion is extremely poisonous. Soluble cyanide salts such as sodium cyanide (NaCN), potassium cyanide (KCN) and tetraethylammonium cyanide () are highly toxic. Covalent cyanides contain the group, and are usually called nitriles if the group is linked by a single covalent bond to carbon atom. For example, in acetonitrile , the cyanide group is bonded to methyl . In tetracyanomethane , four cyano groups are bonded to carbon. Although nitriles generally do not release cyanide ions, the cyanohydrins do and are thus toxic. The cyano group may be covalently bonded to atoms different than carbon, e.g., in cyanogen azide , phosphorus tricyanide and trimethylsilyl cyanide . Hydrogen cyanide, or , is a highly volatile toxic liquid tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. Etymology Because it was produced from halite, rock salt according to the methods of Johann Rudolph Glauber, hydrochloric acid was historically called by European alchemists ''spirits of salt'' or ''acidum salis'' (salt acid). Both names are still used, especially in other languages, such as , , , , , , , , , , (''ensan''), zh, 盐酸 (''yánsuān''), and (''yeomsan''). Gaseous HCl was called ''marine acid air''. The name ''muriatic acid'' has the same origin (''muriatic'' means "pertaining to brine or salt", hence ''muriate'' means hydrochloride), and this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |