fat interesterification on:
[Wikipedia]
[Google]
[Amazon]
In the food industry and
 Enzymatic interesterification (IE) uses an
Enzymatic interesterification (IE) uses an
"Legal and Policy Resources on Public Health 'Winnable Battles'"
www.cdc.gov. Retrieved October 20, 2010. Adoption was greatly facilitated by the development of enzymes bound to inert solid substrates like
T. L. Husum, L. S. Pedersen, P. M. Nielsen, M. W. Christensen, D. Kristensen, and H. C. Holm (2003):
Enzymatic interesterification: Process advantages and product benefits.
. Palm Oil Information Online Service. Retrieved 2010-10-20. W. Hamm and R. Hamilton, editors (2000): ''Edible Oil Processing''. Rousseau, D. (2002): "The Effects of Interesterification on the Physical Properties of Fats". Chapter 13 of ''Physical Properties of Lipids''. CRC Press. “Chemical vs. Enzymatic Interesterification.”
De Greyt, Wim. IUPAC-AOCS Workshop on Fats, Oils & Oilseeds Analyses & Production, 6 Dec. 2004. Retrieved October 20, 2010 {{Cite web , url=http://www.soci.org/-/media/Files/Lecture-Series/pb82.ashx/ , title=Interesterification Process Conditions , last=Kellens , first=Marc , year=2000 , access-date=2007-01-29
Novozymes
Lipids Triglycerides
biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and ...
, interesterification (IE) is a process that rearranges the fatty acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, ...
s of a fat
In nutrition, biology, and chemistry, fat usually means any ester of fatty acids, or a mixture of such compounds, most commonly those that occur in living beings or in food.
The term often refers specifically to triglycerides (triple est ...
product, typically a mixture of triglyceride
A triglyceride (TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids (from ''wikt:tri-#Prefix, tri-'' and ''glyceride'').
Triglycerides are the main constituents of body fat in humans and other ...
. The process implies breaking and reforming the ester bond
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
s C–O–C that connect the fatty acid chains to the glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known ...
hubs of the fat molecules. These reactions are performed by inorganic catalysts, yielding what is called chemical interesterification (CIE) in the industry; or by enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
s, in the so-called enzymatic interesterification (EIE).
This process is typically used to adjust the physical characteristics of the fat, such as melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depen ...
and plasticity, for specific uses. It can be used, for instance, to turn oil
An oil is any nonpolar chemical substance that is composed primarily of hydrocarbons and is hydrophobic (does not mix with water) & lipophilic (mixes with other oils). Oils are usually flammable and surface active. Most oils are unsaturated ...
s into solid or semisolid products by combining them with other solid fats. It can also be used to prevent separation of solid fractions in palm oil and lauric fats, slow rancidification
Rancidification is the process of complete or incomplete autoxidation or hydrolysis of fats and oils when exposed to air, light, moisture, or bacterial action, producing short-chain aldehydes, ketones and free fatty acids.
When these processes o ...
, or create oils more suitable for deep frying.
Compared to other processes that are used for the same purpose, such as hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organ ...
, interesterification generally preserves the original distribution of fatty acids in the product and hence is expected to preserve its nutritional and health attributes. However, those other techniques may still be applied to the starting fats or to the products of IE, and the latter may be blended with other fats. Also, some of the new triglycerides produced by IE may be fractionated
Fractionation is a separation process in which a certain quantity of a mixture (of gases, solids, liquids, enzymes, or isotopes, or a suspension) is divided during a phase transition, into a number of smaller quantities ( fractions) in which t ...
(separated) through controlled crystallization.
Interesterified fats are used in many industrial food products, including cookies, crackers, biscuits, cakes and icings, dairy fat replacers, pie crust, popcorn, flatbread and tortilla
A tortilla (, ) is a thin, circular unleavened flatbread originally made from maize hominy meal, and now also from wheat flour. The Aztecs and other Nahuatl speakers called tortillas ''tlaxcalli'' (). First made by the indigenous peoples of M ...
s.
Feedstock
Typically the feedstock (starting product) is a mixture of two or more oils. In particular, unsaturated vegetable oil can be interesterified with a fully hydrogenated version thereof, as in the illustration to the right. This procedure yields less saturated fat without creating the '' trans'' fat that would be produced by partial hydrogenation. The reaction does not go to completion, and the product will be a mixture of triglycerides with different amounts of saturation.Process
Chemistry
In principle, when interesterification is applied to two pure triglycerides, each with three identical fatty acids (AAA and BBB), the result could contain six different triglycerides (AAA, AAB, ABA, ABB, BAB, and BBB). The number is 6, rather than 23 = 8, because of the symmetry of the glycerol backbone. The number is much greater if the feedstock has three or more distinct fatty acids."Chemical" interesterification
In the so-called "chemical" interesterification, the catalyst is an inorganic compound such assodium methoxide
Sodium methoxide is the simplest sodium alkoxide. With the formula , it is a white solid, which is formed by the deprotonation of methanol. Itis a widely used reagent in industry and the laboratory. It is also a dangerously caustic base.
P ...
. The reaction is carried out at high temperatures and creates three by-products — sodium soap
Soap is a salt of a fatty acid used in a variety of cleansing and lubricating products. In a domestic setting, soaps are surfactants usually used for washing, bathing, and other types of housekeeping. In industrial settings, soaps are use ...
s, fatty methyl ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
s, and monoglyceride
Monoglycerides (also: acylglycerols or monoacylglycerols) are a class of glycerides which are composed of a molecule of glycerol linked to a fatty acid via an ester bond. As glycerol contains both primary and secondary alcohol groups two differen ...
s) in addition to the interesterified fats.
Enzymatic interesterification
 Enzymatic interesterification (IE) uses an
Enzymatic interesterification (IE) uses an enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
to break and reform the ester bonds. Enzymes most suitable for this process are esterase
An esterase is a hydrolase enzyme that splits esters into an acid and an alcohol in a chemical reaction with water called hydrolysis.
A wide range of different esterases exist that differ in their substrate specificity, their protein structure ...
; lipase; acylase; those enzymes that facilitate acidolysis reactions, transesterification
In organic chemistry, transesterification is the process of exchanging the organic group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. The reaction ca ...
reactions, ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides a ...
synthesis or ester interchange reactions; enzymes having phospholipase
A phospholipase is an enzyme that hydrolyzes phospholipids into fatty acids and other lipophilic substances. Acids trigger the release of bound calcium from cellular stores and the consequent increase in free cytosolic Ca2+, an essential step in ...
or protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the ...
activity, including thermostable
In materials science and molecular biology, thermostability is the ability of a substance to resist irreversible change in its chemical or physical structure, often by resisting decomposition or polymerization, at a high relative temperature.
...
and thermotolerant hydrolase
Hydrolase is a class of enzyme that commonly perform as biochemical catalysts that use water to break a chemical bond, which typically results in dividing a larger molecule into smaller molecules. Some common examples of hydrolase enzymes are este ...
activity; and polynucleotide
A polynucleotide molecule is a biopolymer composed of 13 or more nucleotide monomers covalently bonded in a chain. DNA (deoxyribonucleic acid) and RNA (ribonucleic acid) are examples of polynucleotides with distinct biological function. The pre ...
s.
Some enzymes will break and reform ester bonds only at positions 1 and 3 (sp1 and sp3) of the glycerol hub, leaving the acids in position 2 (sp2) fixed.
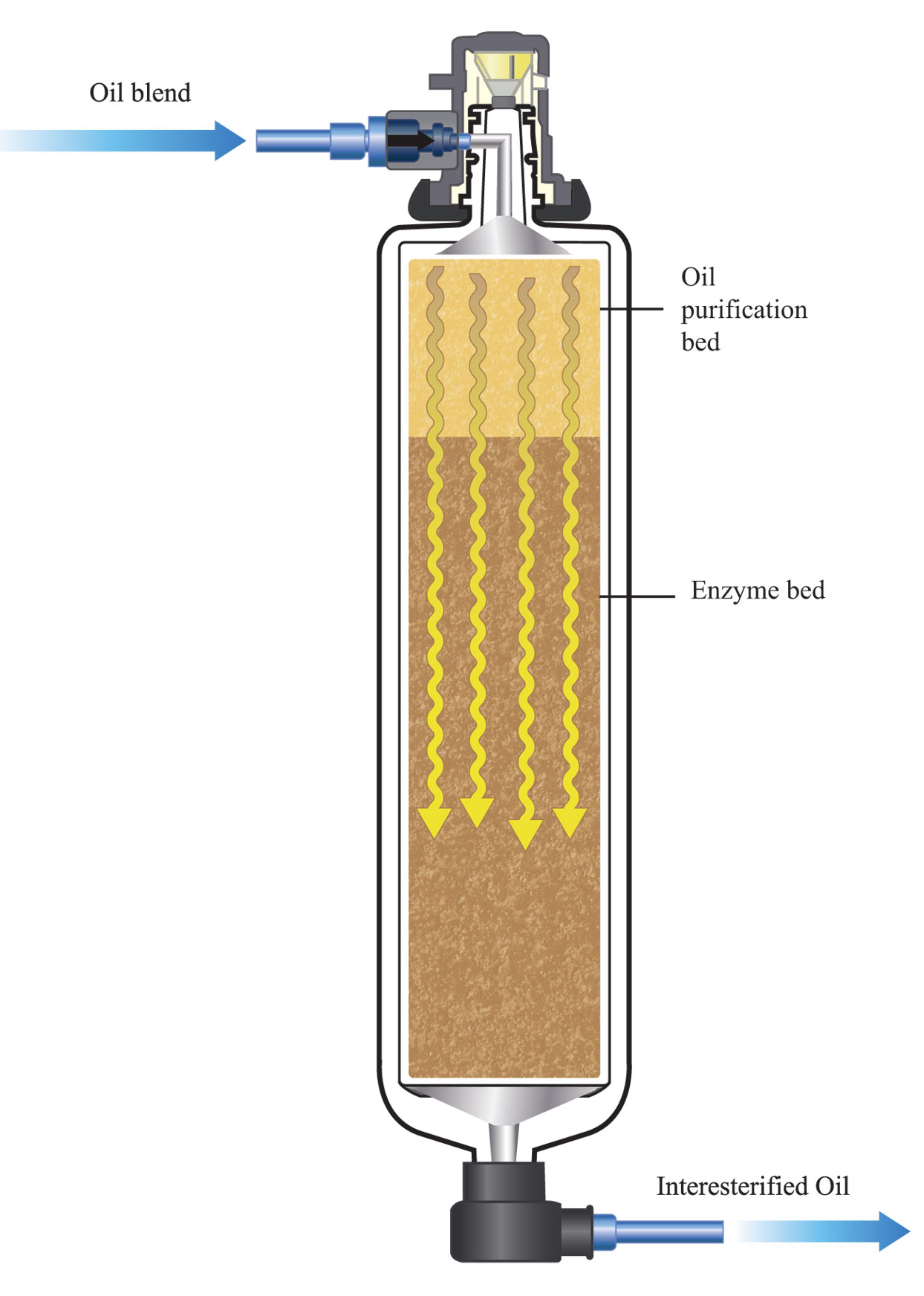
The most common industrial EIE process forces the liquid fat feedstock through a fixed-bed reactor, that typically contains an oil purification bed followed by an enzyme bed. The latter has the enzyme fixed on some inert granular substrate. The first bed removes impurities from the oil blend that could inactivate the enzyme or affect its performance. The enzyme activity decreases over time, so flow must be carefully monitored and adjusted over time to maintain conversion.
Two or more reactors may be used in tandem, where the first reactor has the lowest enzyme activity and absorbs most of the impurities and harmful compounds. This sequencing protects the most active enzymes, which are in the last reactors.
IE has been replacing CIE because it has fewer processing steps, can be carried out at lower temperatures, produces no by-products, and has lower production costs.
Advantages
Compared to simple blends, interesterified fats have a wider plasticity range, meaning that they retain their physical properties over a wider temperature range, without separation of their components. IE can also use a wider variety of feedstocks, such assoybean oil
Soybean oil (British English: soyabean oil) is a vegetable oil extracted from the seeds of the soybean (''Glycine max''). It is one of the most widely consumed cooking oils and the second most consumed vegetable oil. As a drying oil, processed s ...
, they provide a better risk management profile than globally produced palm oil.
History
The earliest record of enzymatic Interesterification was in 1844, whenThéophile-Jules Pelouze
Théophile-Jules Pelouze (also known as Jules Pelouze, Théophile Pelouze, Theo Pelouze, or T. J. Pelouze, ; 26 February 180731 May 1867) was a French chemist.
Life
He was born at Valognes, and died in Paris.
His father, Edmond Pelouze, was an ...
published a study on the synthesis of a triglyceride through the esterification of glycerol by butyric acid. In 1920, Wilhelm Normann
Wilhelm Normann (16 January 1870, in Petershagen – 1 May 1939, in Chemnitz) (sometimes also spelled ''Norman'') was a German chemist who introduced the hydrogenation of fats in 1901, creating what later became known as trans fats. This inventi ...
, who also patented the catalytic hydrogenation of fatty acids, was granted a patent
A patent is a type of intellectual property that gives its owner the legal right to exclude others from making, using, or selling an invention for a limited period of time in exchange for publishing an enabling disclosure of the invention."A ...
for the chemical interesterification of edible lipids. This process became a viable option for the food industry as it improved the spreadability and baking properties of the common shortening lard.
Enzymatic interesterification was developed in the 1970s by the team at the Unilever Research Center at Colworth House in England. Their work proved that the use of a specific enzyme predictably rearranged the fatty acids on the glycerol backbone of a triglyceride at positions 1 and 3. This provided an expanded range of available triglyceride types.
Still, EIE remained largely confined to research laboratories due to high enzyme prices. It was only in the 2000s that general concerns about the health effects of ''trans'' fats drove the industry to adopt interesterification as a replacement for partial hydrogenation (which had been the oil hardening method of choice, due to its lower cost).www.cdc.gov. Retrieved October 20, 2010.
silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is ...
, by Novozymes
Novozymes A/S is a global biotechnology company headquartered in Bagsværd outside of Copenhagen, Denmark. The company's focus is the research, development and production of industrial enzymes, microorganisms, and biopharmaceutical ingredients. ...
and other companies.
References
Enzymatic interesterification: Process advantages and product benefits.
. Palm Oil Information Online Service. Retrieved 2010-10-20. W. Hamm and R. Hamilton, editors (2000): ''Edible Oil Processing''. Rousseau, D. (2002): "The Effects of Interesterification on the Physical Properties of Fats". Chapter 13 of ''Physical Properties of Lipids''. CRC Press. “Chemical vs. Enzymatic Interesterification.”
De Greyt, Wim. IUPAC-AOCS Workshop on Fats, Oils & Oilseeds Analyses & Production, 6 Dec. 2004. Retrieved October 20, 2010 {{Cite web , url=http://www.soci.org/-/media/Files/Lecture-Series/pb82.ashx/ , title=Interesterification Process Conditions , last=Kellens , first=Marc , year=2000 , access-date=2007-01-29
External links
Novozymes
Lipids Triglycerides