A depletion force is an effective attractive force that arises between large
colloidal
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend ...
particles that are suspended in a dilute solution of ''depletants'', which are smaller solutes that are preferentially excluded from the vicinity of the large particles.
One of the earliest reports of depletion forces that lead to particle coagulation is that of Bondy, who observed the separation or "creaming" of rubber latex upon addition of polymer depletant molecules (
sodium alginate
Alginic acid, also called algin, is a naturally occurring, edible polysaccharide found in brown algae. It is hydrophilic and forms a viscous gum when hydrated. With metals such as sodium and calcium, its salts are known as alginates. Its colour ...
) to solution.
More generally, depletants can include
polymers,
micelles
A micelle () or micella () (plural micelles or micellae, respectively) is an aggregate (or supramolecular assembly) of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension (also known as associated coll ...
,
osmolytes
Osmolytes are low-molecular weight organic compounds that influence the properties of biological fluids. Their primary role is to maintain the integrity of cells by affecting the viscosity, melting point, and ionic strength of the aqueous solution. ...
, ink, mud, or paint dispersed in a
continuous phase
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend ...
.
Depletion forces are often regarded as
entropic forces, as was first explained by the established Asakura–Oosawa model.
[Asakura">] In this theory the depletion force arises from an increase in osmotic pressure of the surrounding solution when colloidal particles get close enough such that the excluded cosolutes (depletants) cannot fit in between them.
Because the particles were considered as hard-core (completely rigid) particles, the emerging picture of the underlying mechanism inducing the force was necessarily entropic.
Causes
Sterics
The system of colloids and depletants in solution is typically modeled by treating the large colloids and small depletants as dissimilarly sized
hard spheres
Hard spheres are widely used as model particles in the statistical mechanical theory of fluids and solids. They are defined simply as impenetrable spheres that cannot overlap in space. They mimic the extremely strong ("infinitely elastic bouncing" ...
.
Hard spheres are characterized as non-interacting and impenetrable spheres. These two fundamental properties of hard spheres are described mathematically by the ''hard-sphere potential''. The hard-sphere potential imposes steric constraint around large spheres which in turn gives rise to ''excluded volume'', that is, volume that is unavailable for small spheres to occupy.
Hard-sphere potential
In a colloidal dispersion, the colloid-colloid interaction potential is approximated as the interaction potential between two hard spheres. For two hard spheres of diameter of
, the interaction potential as a function of interparticle separation is:
:
called the hard-sphere potential where
is the center-to-center distance between the spheres.
If both colloids and depletants are in a
dispersion
Dispersion may refer to:
Economics and finance
*Dispersion (finance), a measure for the statistical distribution of portfolio returns
*Price dispersion, a variation in prices across sellers of the same item
*Wage dispersion, the amount of variatio ...
, there is interaction potential between colloidal particles and depletant particles that is described similarly by the hard-sphere potential.
Again, approximating the particles to be hard-spheres, the interaction potential between colloids of diameter
and depletant sols of diameter
is:
:
where
is the center-to-center distance between the spheres. Typically, depletant particles are very small compared to the colloids so
The underlying consequence of the hard-sphere potential is that dispersed colloids cannot penetrate each other and have no mutual attraction or repulsion.
Excluded volume
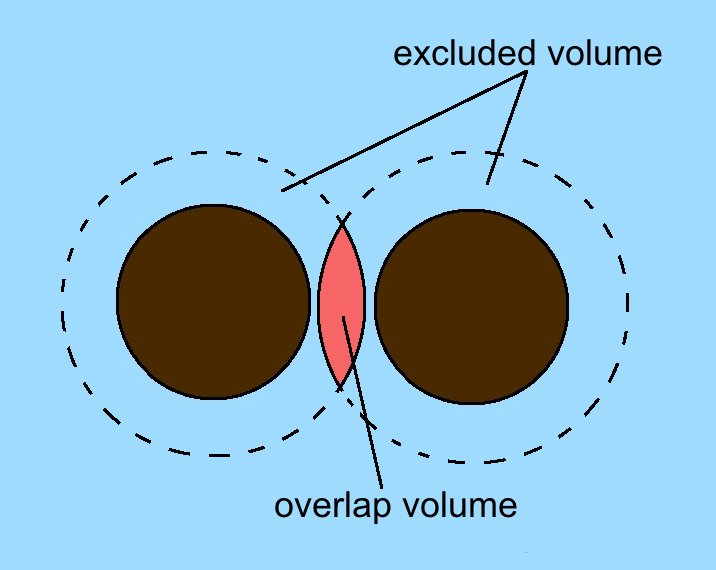
When both large colloidal particles and small depletants are in a
suspension, there is a region which surrounds every large colloidal particle that is unavailable for the centers of the depletants to occupy. This steric restriction is due to the colloid-depletant hard-sphere potential.
The volume of the excluded region is
:
where
is the diameter of the large spheres and
is the diameter of the small spheres.
When the large spheres get close enough, the excluded volumes surrounding the spheres intersect. The overlapping volumes result in a reduced excluded volume, that is, an increase in the total free volume available to small spheres.
The reduced excluded volume,
can be written
:
 When both large colloidal particles and small depletants are in a suspension, there is a region which surrounds every large colloidal particle that is unavailable for the centers of the depletants to occupy. This steric restriction is due to the colloid-depletant hard-sphere potential. The volume of the excluded region is
:
where is the diameter of the large spheres and is the diameter of the small spheres.
When the large spheres get close enough, the excluded volumes surrounding the spheres intersect. The overlapping volumes result in a reduced excluded volume, that is, an increase in the total free volume available to small spheres. The reduced excluded volume, can be written
:
When both large colloidal particles and small depletants are in a suspension, there is a region which surrounds every large colloidal particle that is unavailable for the centers of the depletants to occupy. This steric restriction is due to the colloid-depletant hard-sphere potential. The volume of the excluded region is
:
where is the diameter of the large spheres and is the diameter of the small spheres.
When the large spheres get close enough, the excluded volumes surrounding the spheres intersect. The overlapping volumes result in a reduced excluded volume, that is, an increase in the total free volume available to small spheres. The reduced excluded volume, can be written
: When both large colloidal particles and small depletants are in a suspension, there is a region which surrounds every large colloidal particle that is unavailable for the centers of the depletants to occupy. This steric restriction is due to the colloid-depletant hard-sphere potential. The volume of the excluded region is
:
where is the diameter of the large spheres and is the diameter of the small spheres.
When the large spheres get close enough, the excluded volumes surrounding the spheres intersect. The overlapping volumes result in a reduced excluded volume, that is, an increase in the total free volume available to small spheres. The reduced excluded volume, can be written
:
When both large colloidal particles and small depletants are in a suspension, there is a region which surrounds every large colloidal particle that is unavailable for the centers of the depletants to occupy. This steric restriction is due to the colloid-depletant hard-sphere potential. The volume of the excluded region is
:
where is the diameter of the large spheres and is the diameter of the small spheres.
When the large spheres get close enough, the excluded volumes surrounding the spheres intersect. The overlapping volumes result in a reduced excluded volume, that is, an increase in the total free volume available to small spheres. The reduced excluded volume, can be written
: