|
Conformational Entropy
In chemical thermodynamics, conformational entropy is the entropy associated with the number of conformations of a molecule. The concept is most commonly applied to biological macromolecules such as proteins and RNA, but also be used for polysaccharides and other molecules. To calculate the conformational entropy, the possible conformations of the molecule may first be discretized into a finite number of states, usually characterized by unique combinations of certain structural parameters, each of which has been assigned an energy. In proteins, backbone dihedral angles and side chain rotamers are commonly used as parameters, and in RNA the base pairing pattern may be used. These characteristics are used to define the degrees of freedom (in the statistical mechanics sense of a possible "microstate"). The conformational entropy associated with a particular structure or state, such as an alpha-helix, a folded or an unfolded protein structure, is then dependent on the probabil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Thermodynamics
Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the ''spontaneity'' of processes. The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the "fundamental equations of Gibbs" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics. History In 1865, the German physicist Rudolf Clausius, in his ''Mechanical Theory of Heat'', suggested that the principles o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tertiary Structure
Protein tertiary structure is the three dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains may interact and bond in a number of ways. The interactions and bonds of side chains within a particular protein determine its tertiary structure. The protein tertiary structure is defined by its atomic coordinates. These coordinates may refer either to a protein domain or to the entire tertiary structure.Branden C. and Tooze J. "Introduction to Protein Structure" Garland Publishing, New York. 1990 and 1991. A number of tertiary structures may fold into a quaternary structure.Kyte, J. "Structure in Protein Chemistry." Garland Publishing, New York. 1995. History The science of the tertiary structure of proteins has progressed from one of hypothesis to one of detailed definition. Although Emil Fischer had suggested proteins were made of poly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Folding
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproducible process, a polypeptide folds into its characteristic three-dimensional structure from a random coil. Each protein exists first as an unfolded polypeptide or random coil after being translated from a sequence of mRNA to a linear chain of amino acids. At this stage the polypeptide lacks any stable (long-lasting) three-dimensional structure (the left hand side of the first figure). As the polypeptide chain is being synthesized by a ribosome, the linear chain begins to fold into its three-dimensional structure. Folding of many proteins begins even during translation of the polypeptide chain. Amino acids interact with each other to produce a well-defined three-dimensional structure, the folded protein (the right hand side of the figure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molten Globule
In molecular biology, the term molten globule (MG) refers to protein states that are more or less compact (hence the "globule"), but are lacking the specific tight packing of amino acid residues which creates the solid state-like tertiary structure of completely folded proteins. It was found, for example, in cytochrome c, which conserves a native-like secondary structure content but without the tightly packed protein interior, under low pH and high salt concentration. For cytochrome c and some other proteins, it has been shown that the molten globule state is a " thermodynamic state" clearly different both from the native and the denatured state, demonstrating for the first time the existence of a third equilibirum (i.e., intermediate) state. The term "molten globule" may be used to describe various types of partially-folded protein states found in slightly denaturing conditions such as low pH (generally pH = 2), mild denaturant, or high temperature. Molten globules are col ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Loop Entropy
Loop entropy is the entropy lost upon bringing together two residues of a polymer within a prescribed distance. For a single loop, the entropy varies logarithmically with the number of residues N in the loop : \Delta S = \alpha k_ \ln N \, where k_ is the Boltzmann constant and \alpha is a coefficient that depends on the properties of the polymer. This entropy formula corresponds to a power-law distribution P \sim N^ for the probability of the residues contacting. The loop entropy may also vary with the position of the contacting residues. Residues near the ends of the polymer are more likely to contact (quantitatively, have a lower \alpha) than those in the middle (i.e., far from the ends), primarily due to excluded volume effects. Wang-Uhlenbeck entropy The loop entropy formula becomes more complicated with multiples loops, but may be determined for a Gaussian polymer using a matrix method developed by Wang and Uhlenbeck. Let there be M contacts among the residues, whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Folding Funnel
The folding funnel hypothesis is a specific version of the energy landscape theory of protein folding, which assumes that a protein's native state corresponds to its free energy minimum under the solution conditions usually encountered in cells. Although energy landscapes may be "rough", with many non-native local minima in which partially folded proteins can become trapped, the folding funnel hypothesis assumes that the native state is a deep free energy minimum with steep walls, corresponding to a single well-defined tertiary structure. The term was introduced by Ken A. Dill in a 1987 article discussing the stabilities of globular proteins. The folding funnel hypothesis is closely related to the hydrophobic collapse hypothesis, under which the driving force for protein folding is the stabilization associated with the sequestration of hydrophobic amino acid side chains in the interior of the folded protein. This allows the water solvent to maximize its entropy, lowering the tot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Configuration Entropy
In statistical mechanics, configuration entropy is the portion of a system's entropy that is related to discrete representative positions of its constituent particles. For example, it may refer to the number of ways that atoms or molecules pack together in a mixture, alloy or glass, the number of conformations of a molecule, or the number of spin configurations in a magnet. The name might suggest that it relates to all possible configurations or particle positions of a system, excluding the entropy of their velocity or momentum, but that usage rarely occurs. Calculation If the configurations all have the same weighting, or energy, the configurational entropy is given by Boltzmann's entropy formula :S = k_B \, \ln W, where ''k''''B'' is the Boltzmann constant and ''W'' is the number of possible configurations. In a more general formulation, if a system can be in states ''n'' with probabilities ''P''''n'', the configurational entropy of the system is given by :S = - k_B \, \sum_ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermostability
In materials science and molecular biology, thermostability is the ability of a substance to resist irreversible change in its chemical or physical structure, often by resisting decomposition or polymerization, at a high relative temperature. Thermostable materials may be used industrially as fire retardants. A ''thermostable plastic'', an uncommon and unconventional term, is likely to refer to a thermosetting plastic that cannot be reshaped when heated, than to a thermoplastic that can be remelted and recast. Thermostability is also a property of some proteins. To be a thermostable protein means to be resistant to changes in protein structure due to applied heat. Thermostable proteins Most life-forms on Earth live at temperatures of less than 50 °C, commonly from 15 to 50 °C. Within these organisms are macromolecules (proteins and nucleic acids) which form the three-dimensional structures essential to their enzymatic activity. Above the native temperature of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L- glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG). Proline is the only proteinogenic secondary amino acid which is a secondary amine, as the nitrogen atom is attached both to the α-carbon and to a chain of three carbons that together form a five-membered ring. History and etymology Proline was first isolated in 1900 by Richard Willstätter who obtained t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per amount of substance, i.e. the pressure–volume product, rather than energy per temperature increment per ''particle''. The constant is also a combination of the constants from Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. It is a physical constant that is featured in many fundamental equations in the physical sciences, such as the ideal gas law, the Arrhenius equation, and the Nernst equation. The gas constant is the constant of proportionality that relates the energy scale in physics to the temperature scale and the scale used for amount of substance. Thus, the value of the gas constant ultimately derives from historical decisions and accidents in the setting of units of energy, temperature and amount of substan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boltzmann Distribution
In statistical mechanics and mathematics, a Boltzmann distribution (also called Gibbs distribution Translated by J.B. Sykes and M.J. Kearsley. See section 28) is a probability distribution or probability measure that gives the probability that a system will be in a certain state as a function of that state's energy and the temperature of the system. The distribution is expressed in the form: :p_i \propto e^ where is the probability of the system being in state , is the energy of that state, and a constant of the distribution is the product of the Boltzmann constant and thermodynamic temperature . The symbol \propto denotes proportionality (see for the proportionality constant). The term ''system'' here has a very wide meaning; it can range from a collection of 'sufficient number' of atoms or a single atom to a macroscopic system such as a natural gas storage tank. Therefore the Boltzmann distribution can be used to solve a very wide variety of problems. The distribut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
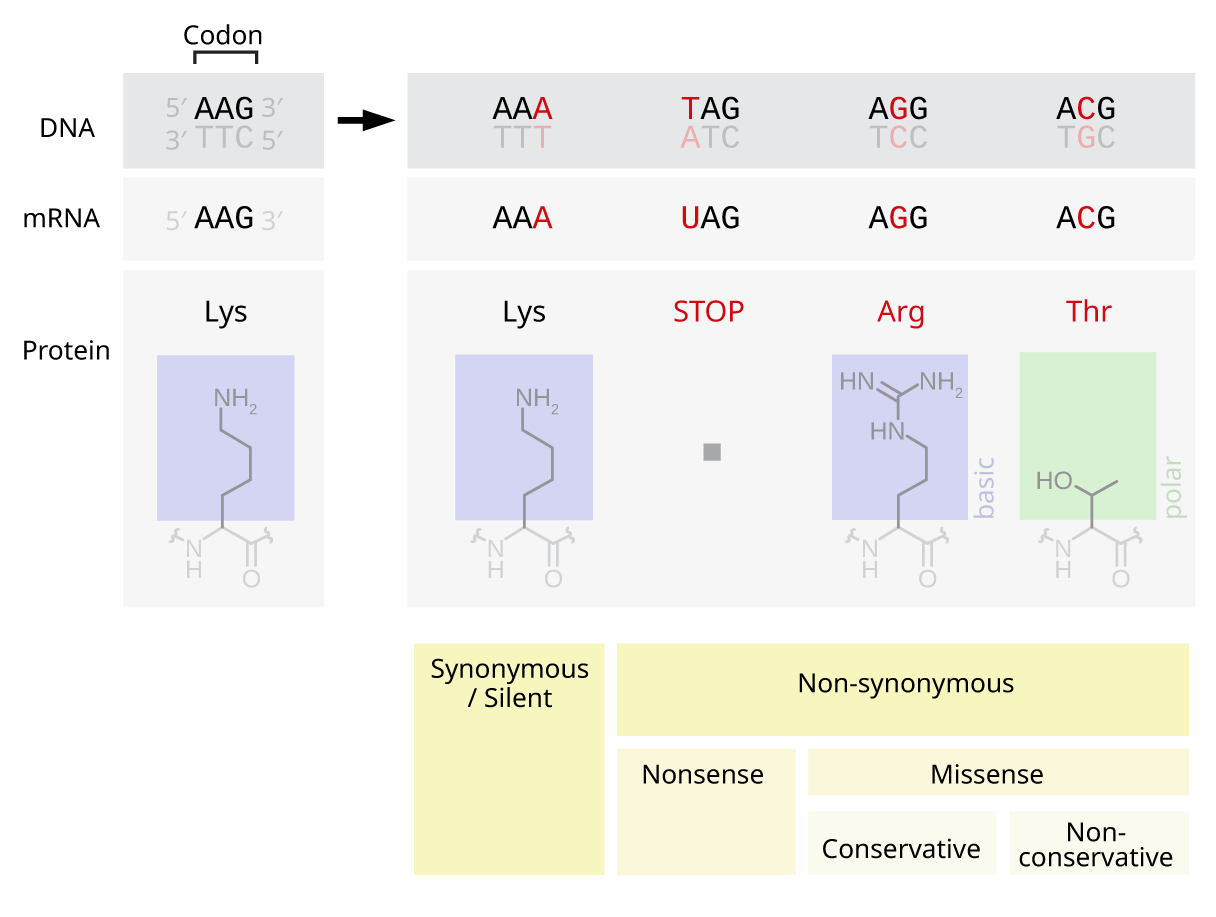
Point Mutation
A point mutation is a genetic mutation where a single nucleotide base is changed, inserted or deleted from a DNA or RNA sequence of an organism's genome. Point mutations have a variety of effects on the downstream protein product—consequences that are moderately predictable based upon the specifics of the mutation. These consequences can range from no effect (e.g. synonymous mutations) to deleterious effects (e.g. frameshift mutations), with regard to protein production, composition, and function. Causes Point mutations usually take place during DNA replication. DNA replication occurs when one double-stranded DNA molecule creates two single strands of DNA, each of which is a template for the creation of the complementary strand. A single point mutation can change the whole DNA sequence. Changing one purine or pyrimidine may change the amino acid that the nucleotides code for. Point mutations may arise from spontaneous mutations that occur during DNA replication. The rate o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |