Zimmerman-Traxler Model on:
[Wikipedia]
[Google]
[Amazon]
The aldol reaction is a means of forming carbon–carbon bonds in organic chemistry.
Discovered independently by the Russian chemist  A typical modern aldol addition reaction, shown above, might involve the
A typical modern aldol addition reaction, shown above, might involve the 

 Acid-catalyzed dehydration
Acid-catalyzed dehydration

 Base-catalyzed dehydration (frequently written incorrectly as a single step, see
Base-catalyzed dehydration (frequently written incorrectly as a single step, see  Although only a catalytic amount of base is required in some cases, the more usual procedure is to use a stoichiometric amount of a strong base such as
Although only a catalytic amount of base is required in some cases, the more usual procedure is to use a stoichiometric amount of a strong base such as

 In this reaction, two unsymmetrical ketones are being condensed using sodium ethoxide. The basicity of sodium ethoxide is such that it cannot fully deprotonate either of the ketones, but can produce small amounts of the sodium enolate of both ketones. This means that, in addition to being potential aldol electrophiles, both ketones may also act as nucleophiles via their sodium enolate. Two electrophiles and two nucleophiles, then, have potential to result in four possible products:
In this reaction, two unsymmetrical ketones are being condensed using sodium ethoxide. The basicity of sodium ethoxide is such that it cannot fully deprotonate either of the ketones, but can produce small amounts of the sodium enolate of both ketones. This means that, in addition to being potential aldol electrophiles, both ketones may also act as nucleophiles via their sodium enolate. Two electrophiles and two nucleophiles, then, have potential to result in four possible products:
 Thus, if one wishes to obtain only one of the cross-products, one must control which carbonyl becomes the nucleophilic enol/enolate and which remains in its electrophilic carbonyl form.
Thus, if one wishes to obtain only one of the cross-products, one must control which carbonyl becomes the nucleophilic enol/enolate and which remains in its electrophilic carbonyl form.
 The malonate is particularly easy to deprotonate because the α position is flanked by more than one carbonyl. Double-activation makes the enolate more stable, so not as strong a base is required to form it. An extension of this effect can allow control over which of the two carbonyl reactants becomes the enolate even if both do have α hydrogens. If one partner is considerably more acidic than the other, the most acidic proton is abstracted by the base and an enolate is formed at that carbonyl while the carbonyl that is less acidic is not affected by the base. This type of control works only if the difference in acidity is large enough and no excess of base is used for the reaction. A typical substrate for this situation is when the deprotonatable position is activated by more than one carbonyl-like group. Common examples include a CH2 group flanked by two carbonyls or nitriles (see for example the Knoevenagel condensation and the first steps of the malonic ester synthesis and acetoacetic ester synthesis).
The malonate is particularly easy to deprotonate because the α position is flanked by more than one carbonyl. Double-activation makes the enolate more stable, so not as strong a base is required to form it. An extension of this effect can allow control over which of the two carbonyl reactants becomes the enolate even if both do have α hydrogens. If one partner is considerably more acidic than the other, the most acidic proton is abstracted by the base and an enolate is formed at that carbonyl while the carbonyl that is less acidic is not affected by the base. This type of control works only if the difference in acidity is large enough and no excess of base is used for the reaction. A typical substrate for this situation is when the deprotonatable position is activated by more than one carbonyl-like group. Common examples include a CH2 group flanked by two carbonyls or nitriles (see for example the Knoevenagel condensation and the first steps of the malonic ester synthesis and acetoacetic ester synthesis).
(2SR,3RS)-2,4-Dimethyl-3-Hydroxypentanoic Acid
, ''
 For deprotonation to occur, the stereoelectronic requirement is that the alpha-C-H sigma bond must be able to overlap with the pi* orbital of the carbonyl:
For deprotonation to occur, the stereoelectronic requirement is that the alpha-C-H sigma bond must be able to overlap with the pi* orbital of the carbonyl:
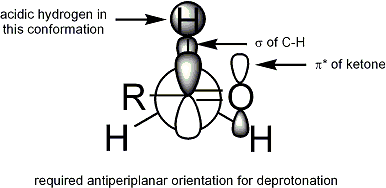
 For ketones, most enolization conditions give ''Z'' enolates. For esters, most enolization conditions give ''E'' enolates. The addition of HMPA is known to reverse the stereoselectivity of deprotonation.
For ketones, most enolization conditions give ''Z'' enolates. For esters, most enolization conditions give ''E'' enolates. The addition of HMPA is known to reverse the stereoselectivity of deprotonation.
 The stereoselective formation of enolates has been rationalized with the Ireland model, although its validity is somewhat questionable. In most cases, it is not known which, if any, intermediates are monomeric or
The stereoselective formation of enolates has been rationalized with the Ireland model, although its validity is somewhat questionable. In most cases, it is not known which, if any, intermediates are monomeric or
 The trisubstituted enolate is considered the
The trisubstituted enolate is considered the
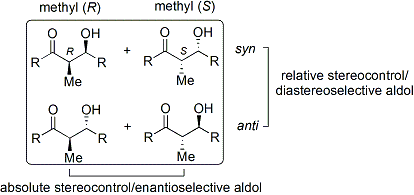
 The convention applies when propionate (or higher order) nucleophiles are added to aldehydes. The ''R'' group of the ketone and the ''R group of the aldehyde are aligned in a "zig zag" pattern in the plane of the paper (or screen), and the disposition of the formed stereocenters is deemed ''syn'' or ''anti'', depending if they are on the same or opposite sides of the main chain.
Older papers use the '' erythro/threo'' nomenclature familiar from saccharide chemistry.
The convention applies when propionate (or higher order) nucleophiles are added to aldehydes. The ''R'' group of the ketone and the ''R group of the aldehyde are aligned in a "zig zag" pattern in the plane of the paper (or screen), and the disposition of the formed stereocenters is deemed ''syn'' or ''anti'', depending if they are on the same or opposite sides of the main chain.
Older papers use the '' erythro/threo'' nomenclature familiar from saccharide chemistry.


 For example, boron–carbon and boron–oxygen bonds are 1.4–1.5 Å and 1.5–1.6 Å in length, respectively, whereas typical metal-carbon and metal-oxygen bonds are 1.9–2.2 Å and 2.0–2.2 Å in length, respectively. The use of boron rather than a metal "tightens" the transition state and gives greater stereoselectivity in the reaction. Thus the above reaction gives a ''syn:anti'' ratio of 80:20 using a lithium enolate compared to 97:3 using a bibutylboron enolate.
For example, boron–carbon and boron–oxygen bonds are 1.4–1.5 Å and 1.5–1.6 Å in length, respectively, whereas typical metal-carbon and metal-oxygen bonds are 1.9–2.2 Å and 2.0–2.2 Å in length, respectively. The use of boron rather than a metal "tightens" the transition state and gives greater stereoselectivity in the reaction. Thus the above reaction gives a ''syn:anti'' ratio of 80:20 using a lithium enolate compared to 97:3 using a bibutylboron enolate.
 In the case of an E enolate, the dominant control element is allylic 1,3-strain whereas in the case of a Z enolate, the dominant control element is the avoidance of 1,3-diaxial interactions. The general model is presented below:
In the case of an E enolate, the dominant control element is allylic 1,3-strain whereas in the case of a Z enolate, the dominant control element is the avoidance of 1,3-diaxial interactions. The general model is presented below:
 For clarity, the stereocenter on the enolate has been epimerized; in reality, the opposite diastereoface of the aldehyde would have been attacked. In both cases, the 1,3-syn diastereomer is favored. There are many examples of this type of stereocontrol:
For clarity, the stereocenter on the enolate has been epimerized; in reality, the opposite diastereoface of the aldehyde would have been attacked. In both cases, the 1,3-syn diastereomer is favored. There are many examples of this type of stereocontrol:

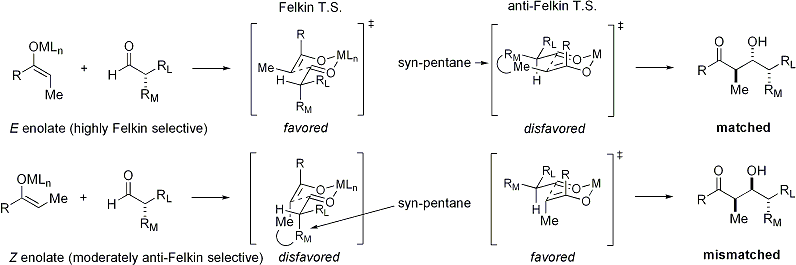 Since ''Z'' enolates must react through a transition state that contains either a destabilizing syn-pentane interaction or an anti-Felkin rotamer, ''Z''-enolates exhibit lower levels of diastereoselectivity in this case. Some examples are presented below:
Since ''Z'' enolates must react through a transition state that contains either a destabilizing syn-pentane interaction or an anti-Felkin rotamer, ''Z''-enolates exhibit lower levels of diastereoselectivity in this case. Some examples are presented below:

Diastereoselective Aldol Condensation Using A Chiral Oxazolidinone Auxiliary: (2S*,3S*)-3-Hydroxy-3-Phenyl-2-Methylpropanoic Acid
, Organic Syntheses, Coll. Vol. 8, p.339 (1993); Vol. 68, p.83 (1990). Developed in the late 1970s and 1980s by
 In the case of the Evans' method, the chiral auxiliary appended is an oxazolidinone, and the resulting carbonyl compound is an
In the case of the Evans' method, the chiral auxiliary appended is an oxazolidinone, and the resulting carbonyl compound is an 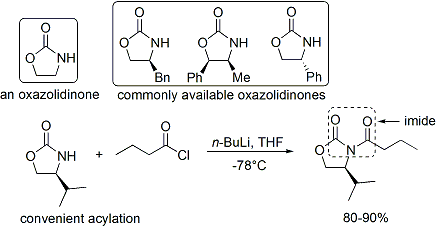 The acylation of an oxazolidinone is a convenient procedure, and is informally referred to as "loading done". ''Z''-enolates, leading to syn-aldol adducts, can be reliably formed using boron-mediated soft enolization:
The acylation of an oxazolidinone is a convenient procedure, and is informally referred to as "loading done". ''Z''-enolates, leading to syn-aldol adducts, can be reliably formed using boron-mediated soft enolization:
 Often, a single diastereomer may be obtained by one
Often, a single diastereomer may be obtained by one  Upon construction of the imide, both syn- and anti-selective aldol addition reactions may be performed, allowing the assemblage of three of the four possible stereoarrays: syn selective: and anti selective:
Upon construction of the imide, both syn- and anti-selective aldol addition reactions may be performed, allowing the assemblage of three of the four possible stereoarrays: syn selective: and anti selective:
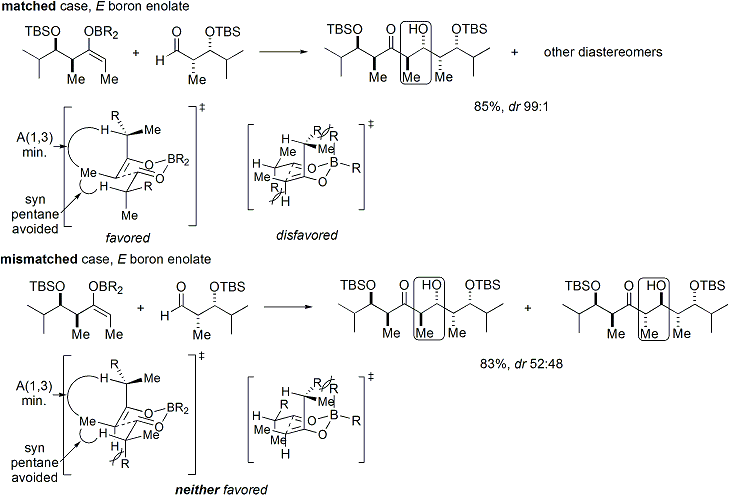
 In the syn-selective reactions, both enolization methods give the ''Z'' enolate, as expected; however, the stereochemical outcome of the reaction is controlled by the methyl stereocenter, rather than the chirality of the oxazolidinone. The methods described allow the stereoselective assembly of polyketides, a class of natural products that often feature the aldol retron.
In the syn-selective reactions, both enolization methods give the ''Z'' enolate, as expected; however, the stereochemical outcome of the reaction is controlled by the methyl stereocenter, rather than the chirality of the oxazolidinone. The methods described allow the stereoselective assembly of polyketides, a class of natural products that often feature the aldol retron.
 Intramolecular aldol reaction is the condensation reaction of two aldehyde groups or
Intramolecular aldol reaction is the condensation reaction of two aldehyde groups or  Intramolecular aldol reactions have been widely used in total syntheses of various natural products, especially
Intramolecular aldol reactions have been widely used in total syntheses of various natural products, especially
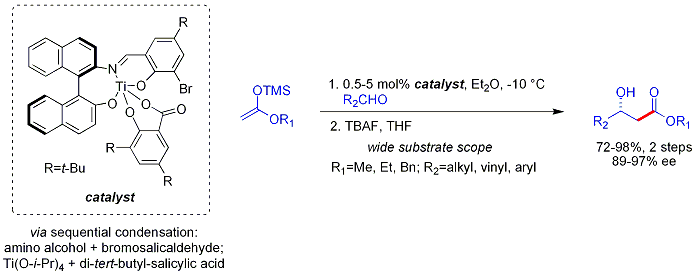 The analogous vinylogous Mukaiyama aldol process can also be rendered catalytic and asymmetric. The example shown below works efficiently for aromatic (but not aliphatic) aldehydes and the mechanism is believed to involve a chiral, metal-bound dienolate.
The analogous vinylogous Mukaiyama aldol process can also be rendered catalytic and asymmetric. The example shown below works efficiently for aromatic (but not aliphatic) aldehydes and the mechanism is believed to involve a chiral, metal-bound dienolate.
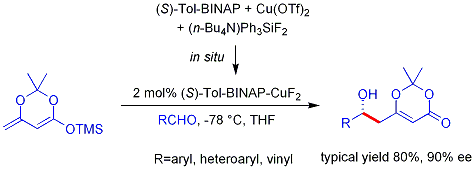

 This reaction is known as the
This reaction is known as the 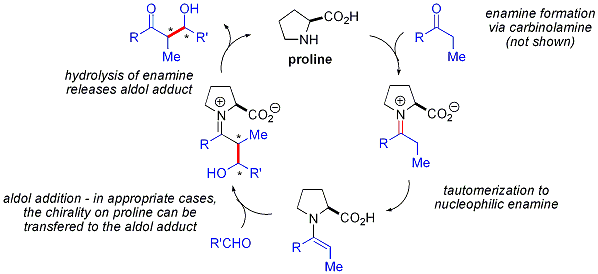 This strategy allows the otherwise challenging cross-aldol reaction between two aldehydes. In general, cross-aldol reactions between aldehydes are typically challenging because they can polymerize easily or react unselectively to give a statistical mixture of products. The first example is shown below:
This strategy allows the otherwise challenging cross-aldol reaction between two aldehydes. In general, cross-aldol reactions between aldehydes are typically challenging because they can polymerize easily or react unselectively to give a statistical mixture of products. The first example is shown below:
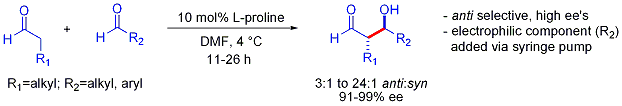 In contrast to the preference for syn adducts typically observed in enolate-based aldol additions, these organocatalyzed aldol additions are anti-selective. In many cases, the organocatalytic conditions are mild enough to avoid polymerization. However, selectivity requires the slow syringe-pump controlled addition of the desired electrophilic partner because both reacting partners typically have enolizable protons. If one aldehyde has no enolizable protons or alpha- or beta-branching, additional control can be achieved.
An elegant demonstration of the power of asymmetric organocatalytic aldol reactions was disclosed by MacMillan and coworkers in 2004 in their synthesis of differentially protected carbohydrates. While traditional synthetic methods accomplish the synthesis of hexoses using variations of iterative protection-deprotection strategies, requiring 8–14 steps, organocatalysis can access many of the same substrates using an efficient two-step protocol involving the proline-catalyzed dimerization of alpha-oxyaldehydes followed by tandem Mukaiyama aldol cyclization.
In contrast to the preference for syn adducts typically observed in enolate-based aldol additions, these organocatalyzed aldol additions are anti-selective. In many cases, the organocatalytic conditions are mild enough to avoid polymerization. However, selectivity requires the slow syringe-pump controlled addition of the desired electrophilic partner because both reacting partners typically have enolizable protons. If one aldehyde has no enolizable protons or alpha- or beta-branching, additional control can be achieved.
An elegant demonstration of the power of asymmetric organocatalytic aldol reactions was disclosed by MacMillan and coworkers in 2004 in their synthesis of differentially protected carbohydrates. While traditional synthetic methods accomplish the synthesis of hexoses using variations of iterative protection-deprotection strategies, requiring 8–14 steps, organocatalysis can access many of the same substrates using an efficient two-step protocol involving the proline-catalyzed dimerization of alpha-oxyaldehydes followed by tandem Mukaiyama aldol cyclization.
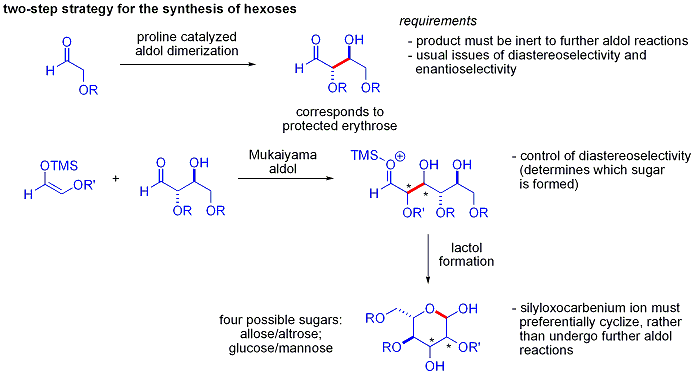 The aldol dimerization of alpha-oxyaldehydes requires that the aldol adduct, itself an aldehyde, be inert to further aldol reactions.
Earlier studies revealed that aldehydes bearing alpha-alkyloxy or alpha-
The aldol dimerization of alpha-oxyaldehydes requires that the aldol adduct, itself an aldehyde, be inert to further aldol reactions.
Earlier studies revealed that aldehydes bearing alpha-alkyloxy or alpha-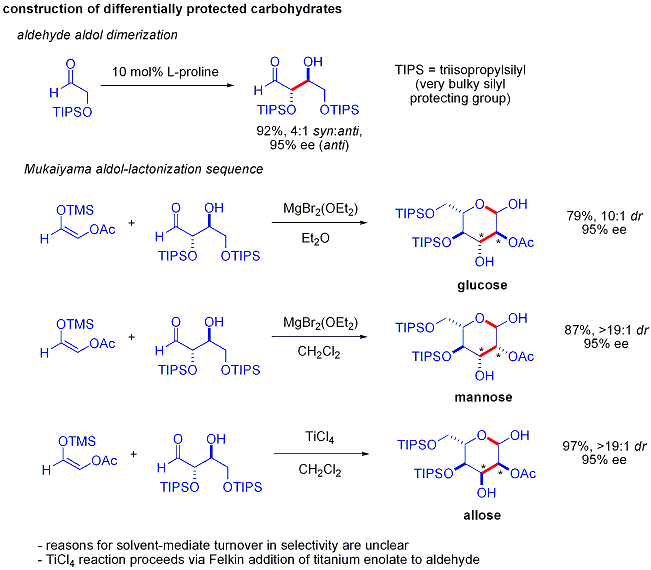
 One approach, demonstrated by Evans, is to silylate the aldol adduct. A silicon reagent such as TMSCl is added in the reaction, which replaces the metal on the alkoxide, allowing
One approach, demonstrated by Evans, is to silylate the aldol adduct. A silicon reagent such as TMSCl is added in the reaction, which replaces the metal on the alkoxide, allowing 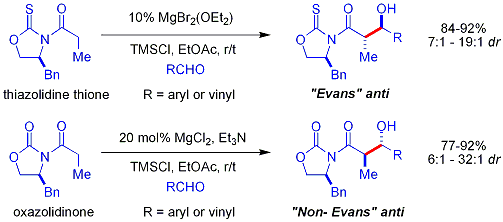
Alexander Borodin
Alexander Porfiryevich Borodin ( rus, link=no, Александр Порфирьевич Бородин, Aleksandr Porfir’yevich Borodin , p=ɐlʲɪkˈsandr pɐrˈfʲi rʲjɪvʲɪtɕ bərɐˈdʲin, a=RU-Alexander Porfiryevich Borodin.ogg, ...
in 1869 and by the French chemist Charles-Adolphe Wurtz
Charles Adolphe Wurtz (; 26 November 181710 May 1884) was an Alsatian French chemist. He is best remembered for his decades-long advocacy for the atomic theory and for ideas about the structures of chemical compounds, against the skeptical opinio ...
in 1872, the reaction combines two carbonyl compounds (the original experiments used aldehydes) to form a new β-hydroxy carbonyl compound. These products are known as '' aldols'', from the ''ald''ehyde + alcoh''ol'', a structural motif seen in many of the products. Aldol structural units are found in many important molecules, whether naturally occurring or synthetic.
For example, the aldol reaction has been used in the large-scale production of the commodity chemical pentaerythritol
and the synthesis of the heart disease drug Lipitor ( atorvastatin, calcium salt).
The aldol reaction unites two relatively simple molecules
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
into a more complex one. Increased complexity arises because up to two new stereogenic center
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups cr ...
s (on the α- and β-carbon of the aldol adduct, marked with asterisks in the scheme below) are formed. Modern methodology is capable of not only allowing aldol reactions to proceed in high yield but also controlling both the relative and absolute configuration of these stereocenters. This ability to selectively synthesize a particular stereoisomer is significant because stereoisomers can have distinctive chemical and biological properties.
For example, stereogenic aldol units are especially common in polyketides, a class of molecules found in biological organisms. In nature, polyketides are synthesized by enzymes
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecule ...
that effect iterative Claisen condensations. The 1,3-dicarbonyl products of these reactions can then be variously derivatized to produce a wide variety of interesting structures. Often, such derivitization involves the reduction of one of the carbonyl groups, producing the aldol subunit. Some of these structures have potent biological properties: the immunosuppressant FK506, the anti-tumor agent discodermolide, or the antifungal agent
An antifungal medication, also known as an antimycotic medication, is a pharmaceutical fungicide or fungistatic used to treat and prevent mycosis such as athlete's foot, ringworm, candidiasis (thrush), serious systemic infections such as crypto ...
amphotericin B, for example. Although the synthesis of many such compounds was once considered nearly impossible, aldol methodology has allowed their efficient synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
*Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
** Organic synthesis, the chemical synthesis of organ ...
in many cases.
nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions di ...
of a ketone enolate
A ketone enolate is an enolate formed from a ketone by reaction with a strong base, such as lithium diisopropylamide, or with a Lewis acid such as dibutylboron trifluoromethanesulfonate and a weak base such as N,N-diisopropylethylamine, ''N'',''N'' ...
to an aldehyde. Once formed, the aldol product can sometimes lose a molecule of water to form an α,β-unsaturated carbonyl compound. This is called '' aldol condensation''. A variety of nucleophiles may be employed in the aldol reaction, including the enols, enolates, and enol ethers of ketones, aldehydes, and many other carbonyl compounds. The electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carri ...
partner is usually an aldehyde or ketone (many variations, such as the Mannich reaction, exist). When the nucleophile and electrophile are different, the reaction is called a ''crossed aldol reaction''; on the converse, when the nucleophile and electrophile are the same, the reaction is called an ''aldol dimerization
A dimer () (''wikt:di-, di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, Covalent bond, covalent or Intermolecular force, intermolecular. Dimers also have significant im ...
''.

Mechanisms
The aldol reaction may proceed by two distinct mechanisms. Carbonyl compounds, such as aldehydes and ketones, can be converted to enols or enol ethers. These species, being nucleophilic at the α-carbon, can attack especially reactive protonated carbonyls such as protonated aldehydes. This is the 'enol mechanism'. Carbonyl compounds, being carbon acids, can also be deprotonated to form enolates, which are much more nucleophilic than enols or enol ethers and can attack electrophiles directly. The usual electrophile is an aldehyde, since ketones are much less reactive. This is the 'enolate mechanism'. Despite the attractiveness of the aldol manifold, there are several problems that need to be addressed to render the process catalytic and effective. The first problem is a thermodynamic one: most aldol reactions are reversible. Furthermore, the equilibrium is also just barely on the side of the products in the case of simple aldehyde–ketone aldol reactions. If the conditions are particularly harsh (e.g.: NaOMe/MeOH/ reflux), condensation may occur, but this can usually be avoided with mild reagents and low temperatures (e.g., LDA (a strong base), THF, −78 °C). Although the aldol addition usually proceeds to near completion under irreversible conditions, the isolated aldol adducts are sensitive to base-induced retro-aldol cleavage to return starting materials. In contrast, retro-aldol condensations are rare, but possible. This is the basis of the catalytic strategy of class I aldolases in nature, as well as numerous small-molecule amine catalysts.Enol mechanism
When an acid catalyst is used, the initial step in thereaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ...
involves acid-catalyzed tautomerization of the carbonyl compound to the enol. The acid also serves to activate the carbonyl group of ''another molecule'' by protonation, rendering it highly electrophilic. The enol is nucleophilic at the α-carbon, allowing it to attack the protonated carbonyl compound, leading to the aldol after deprotonation. This usually dehydrates to give the unsaturated carbonyl compound. The scheme shows a typical acid-catalyzed self-condensation of an aldehyde.
Acid-catalyzed aldol mechanism
Enolate mechanism
If the catalyst is a moderate base such as hydroxide ion or analkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, whe ...
, the aldol reaction occurs via nucleophilic attack by the resonance-stabilized enolate on the carbonyl group of another molecule. The product is the alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, whe ...
salt of the aldol product. The aldol itself is then formed, and it may then undergo dehydration to give the unsaturated carbonyl compound. The scheme shows a simple mechanism for the base-catalyzed aldol reaction of an aldehyde with itself.
Base-catalyzed aldol reaction (shown using −OCH3 as base)
E1cB elimination reaction
The E1cB elimination reaction is a type of elimination reaction which occurs under basic conditions, where the hydrogen to be removed is relatively acidic, while the leaving group (such as -OH or -OR) is a relatively poor one. Usually a moderate t ...
)
LDA LDA may refer to:
Aviation
*Localizer type directional aid, an instrument approach to an airport
*Landing distance available, the length of runway that is available for the ground run of an airplane landing
Law
*Legal document assistant, a non-la ...
or NaHMDS. In this case, enolate formation is irreversible, and the aldol product is not formed until the metal alkoxide of the aldol product is protonated in a separate workup step.
Zimmerman–Traxler model
More refined forms of the mechanism are known. In 1957,Howard Zimmerman
Howard E. Zimmerman (July 5, 1926 – February 12, 2012) was a professor of chemistry at the University of Wisconsin–Madison. He was elected to the National Academy of Sciences in 1980 and the recipient of the 1986 American Institute of Chemist ...
and Marjorie D. Traxler proposed that some aldol reactions have "six-membered transition states having a chair conformation." This is now known as the Zimmerman–Traxler model. ''E''-enolates give rise to anti products, whereas ''Z''-enolates give rise to syn products. The factors that control selectivity are the preference for placing substituents equatorially in six-membered transition states and the avoidance of syn-pentane interactions, respectively. E and Z
E, or e, is the fifth letter and the second vowel letter in the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is ''e'' (pronounced ); pl ...
refer to the cis-trans stereochemical relationship between the enolate oxygen bearing the positive counterion and the highest priority group on the alpha carbon. In reality, only some metals such as lithium reliably follow the Zimmerman–Traxler model. Thus, in some cases, the stereochemical outcome of the reaction may be unpredictable.
Crossed-aldol reactant control
The problem of "control" in the aldol addition is best demonstrated by an example. Consider the outcome of this hypothetical reaction:Acidity
The simplest control is if only one of the reactants has acidic protons, and only this molecule forms the enolate. For example, the addition of diethyl malonate intobenzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-like odor. ...
would produce only one product. Only the malonate has α hydrogens, so it is the nucleophilic partner, whereas the non-enolizeable benzaldehyde can only be the electrophile:
Order of addition
One common solution is to form the enolate of one partner first, and then add the other partner under kinetic control.Bal, B.; Buse, C. T.; Smith, K.; Heathcock, C. H.(2SR,3RS)-2,4-Dimethyl-3-Hydroxypentanoic Acid
, ''
Org. Synth.
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and exper ...
'', Coll. Vol. 7, p.185 (1990); Vol. 63, p.89 (1985). Kinetic control means that the forward aldol addition reaction must be significantly faster than the reverse retro-aldol reaction. For this approach to succeed, two other conditions must also be satisfied; it must be possible to quantitatively form the enolate of one partner, and the forward aldol reaction must be significantly faster than the transfer of the enolate from one partner to another. Common kinetic control conditions involve the formation of the enolate of a ketone with LDA LDA may refer to:
Aviation
*Localizer type directional aid, an instrument approach to an airport
*Landing distance available, the length of runway that is available for the ground run of an airplane landing
Law
*Legal document assistant, a non-la ...
at −78 °C, followed by the slow addition of an aldehyde.
Enolates
Formation
The enolate may be formed by using a strong base ("hard conditions", pathway 1 below) or using aLewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
and a weak base ("soft conditions", pathway 2 below):

Geometry
Extensive studies have been performed on the formation of enolates. It is possible to generate, in most cases, the desired enolate geometry:oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relativ ...
ic in nature; nonetheless, the Ireland model remains a useful tool for understanding enolates.
In the Ireland model, the deprotonation is assumed to proceed by a six-membered or cyclic monomeric transition state. The larger of the two substituents on the electrophile (in the case above, methyl is larger than proton) adopts an equatorial disposition in the favored transition state, leading to a preference for E enolates. The model clearly fails in many cases; for example, if the solvent mixture is changed from THF to 23% HMPA-THF (as seen above), the enolate geometry is reversed, which is inconsistent with this model and its cyclic transition state.
Regiochemistry
If an unsymmetrical ketone is subjected to base, it has the potential to form two regioisomeric enolates (ignoring enolate geometry). For example:kinetic
Kinetic (Ancient Greek: κίνησις “kinesis”, movement or to move) may refer to:
* Kinetic theory of gases, Kinetic theory, describing a gas as particles in random motion
* Kinetic energy, the energy of an object that it possesses due to i ...
enolate, while the tetrasubstituted enolate is considered the thermodynamic enolate. The alpha hydrogen deprotonated to form the kinetic enolate is less hindered, and therefore deprotonated more quickly. In general, tetrasubstituted olefins are more stable than trisubstituted olefins due to hyperconjugative stabilization. The ratio of enolate regioisomers is heavily influenced by the choice of base. For the above example, kinetic control may be established with LDA at −78 °C, giving 99:1 selectivity of kinetic: thermodynamic enolate, while thermodynamic control may be established with triphenylmethyllithium at room temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
, giving 10:90 selectivity.
In general, kinetic enolates are favored by cold temperatures, conditions that give relatively ionic metal–oxygen bonding, and rapid deprotonation using a slight excess of a strong, sterically hindered base. The large base only deprotonates the more accessible hydrogen, and the low temperatures and excess base help avoid equilibration to the more stable alternate enolate after initial enolate formation. Thermodynamic enolates are favored by longer equilibration times at higher temperatures, conditions that give relatively covalent metal–oxygen bonding, and use of a slight sub-stoichiometric amount of strong base. By using insufficient base to deprotonate all of the carbonyl molecules, the enolates and carbonyls can exchange protons with each other and equilibrate to their more stable isomer. Using various metals and solvents can provide control over the amount of ionic character in the metal–oxygen bond.
Stereoselectivity
The aldol reaction is particularly useful because two new stereogenic centers are generated in one reaction. The ''syn''/''anti'' convention is commonly used to denote the relative stereochemistry at the α- and β-carbon.Enolate geometry
There is no significant difference between the level ofstereoinduction
In stereochemistry, asymmetric induction (also enantioinduction) describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the s ...
observed with ''E'' and ''Z'' enolates. Each alkene geometry leads primarily to one specific relative stereochemistry in the product, ''E'' giving ''anti'' and ''Z'' giving ''syn'':
Metal ion
The enolate metal cation may play a large role in determining the level of stereoselectivity in the aldol reaction.Boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has th ...
is often used because its bond lengths are significantly shorter than that of metals such as lithium, aluminium, or magnesium.
Alpha stereocenter on the enolate
The aldol reaction may exhibit "substrate-based stereocontrol", in which existingchirality
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
on either reactant influences the stereochemical outcome of the reaction. This has been extensively studied, and in many cases, one can predict the sense of asymmetric induction
In stereochemistry, asymmetric induction (also enantioinduction) describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the sub ...
, if not the absolute level of diastereoselectivity. If the enolate contains a stereocenter in the alpha position, excellent stereocontrol may be realized.
Alpha stereocenter on the electrophile
When enolates attacks aldehydes with an alpha stereocenter, excellent stereocontrol is also possible. The general observation is that ''E'' enolates exhibit Felkin diastereoface selection, while ''Z'' enolates exhibit anti-Felkin selectivity. The general modelEvans D. A. ''et al.'' ''Top. Stereochem.'' 1982, ''13'', 1–115. (Review) is presented below: Since ''Z'' enolates must react through a transition state that contains either a destabilizing syn-pentane interaction or an anti-Felkin rotamer, ''Z''-enolates exhibit lower levels of diastereoselectivity in this case. Some examples are presented below:
Since ''Z'' enolates must react through a transition state that contains either a destabilizing syn-pentane interaction or an anti-Felkin rotamer, ''Z''-enolates exhibit lower levels of diastereoselectivity in this case. Some examples are presented below:

Unified model of stereoinduction
If both the enolate and the aldehyde contain pre-existing chirality, then the outcome of the "double stereodifferentiating" aldol reaction may be predicted using a merged stereochemical model that takes into account the enolate facial bias, enolate geometry, and aldehyde facial bias. Several examples of the application of this model are given below:
Evans' oxazolidinone chemistry
A widely used method is the Evans' acyl oxazolidinone method.Gage J. R.; Evans D. A.Diastereoselective Aldol Condensation Using A Chiral Oxazolidinone Auxiliary: (2S*,3S*)-3-Hydroxy-3-Phenyl-2-Methylpropanoic Acid
, Organic Syntheses, Coll. Vol. 8, p.339 (1993); Vol. 68, p.83 (1990). Developed in the late 1970s and 1980s by
David A. Evans
David A. Evans (January 11, 1941 – April 29, 2022) was an American chemist who was the Abbott and James Lawrence professor of chemistry at Harvard University. He was a prominent figure in the field of organic chemistry and his research focus ...
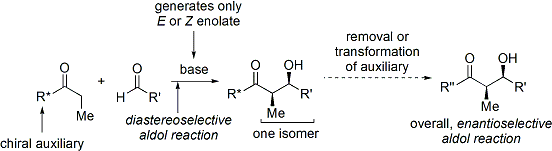
and coworkers, the method works by temporarily creating a chiral enolate by appending a chiral auxiliary. The pre-existing chirality from the auxiliary is then transferred to the aldol adduct by performing a diastereoselective aldol reaction. Upon subsequent removal of the auxiliary, the desired aldol stereoisomer is revealed.
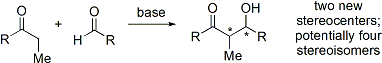

 In the case of the Evans' method, the chiral auxiliary appended is an oxazolidinone, and the resulting carbonyl compound is an
In the case of the Evans' method, the chiral auxiliary appended is an oxazolidinone, and the resulting carbonyl compound is an imide
In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applications, ...
. A number of oxazolidinones are now readily available in both enantiomeric forms. They are relatively expensive. However, enantiopure oxazolidinones are derived in 2 synthetic steps from comparatively inexpensive amino acids, which means that large-scale syntheses can be made more economical by in-house preparation. This usually involves borohydride mediated reduction of the acid moiety
Moiety may refer to:
Chemistry
* Moiety (chemistry), a part or functional group of a molecule
** Moiety conservation, conservation of a subgroup in a chemical species
Anthropology
* Moiety (kinship), either of two groups into which a society is ...
, followed by condensation/cyclisation of the resulting amino alcohol with a simple carbonate ester such as diethylcarbonate.
 The acylation of an oxazolidinone is a convenient procedure, and is informally referred to as "loading done". ''Z''-enolates, leading to syn-aldol adducts, can be reliably formed using boron-mediated soft enolization:
The acylation of an oxazolidinone is a convenient procedure, and is informally referred to as "loading done". ''Z''-enolates, leading to syn-aldol adducts, can be reliably formed using boron-mediated soft enolization:
 Often, a single diastereomer may be obtained by one
Often, a single diastereomer may be obtained by one crystallization
Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposi ...
of the aldol adduct. However, anti-aldol adducts cannot be obtained reliably with the Evans method. Despite the cost and the limitation to give only ''syn'' adducts, the method's superior reliability, ease of use, and versatility render it the method of choice in many situations. Many methods are available for the cleavage of the auxiliary:
 Upon construction of the imide, both syn- and anti-selective aldol addition reactions may be performed, allowing the assemblage of three of the four possible stereoarrays: syn selective: and anti selective:
Upon construction of the imide, both syn- and anti-selective aldol addition reactions may be performed, allowing the assemblage of three of the four possible stereoarrays: syn selective: and anti selective:
Intramolecular reaction
ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
groups in the same molecule. Five- or six-membered , -unsaturated ketone or aldehydes are formed as products. This reaction is an important approach to the formation of carbon-carbon bonds in organic molecules containing ring systems. As an example, under strong basic conditions (e.g. sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
), hexane-2,5-dione
2,5-Hexanedione (Acetonylacetone) is an aliphatic diketone. It is a colorless liquid. In humans, it is a toxic metabolite of hexane and of 2-hexanone.
Symptoms of poisoning
The chronic toxicity of hexane is attributed to hexane-2,5-dione. The ...
(compound A in Figure 1) can cyclize via intramolecular aldol reaction to form the 3-methylcyclopent-2-en-1-one (compound B).
The mechanism of the intramolecular aldol reaction involves formation of a key enolate intermediate followed by an intramolecular nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions di ...
process. First, hydroxide abstracts the α-hydrogen on a terminal carbon to form the enolate. Next, a nucleophilic attack of the enolate on the other keto group forms a new carbon-carbon bond (red) between carbons 2 and 6. At last, usually under heating conditions, the elimination of water molecule yields the cyclized α,β-unsaturated ketone.
alkaloids
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar st ...
and steroids
A steroid is a biologically active organic compound with four rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and a ...
. An example is the application of an intramolecular aldol reaction in the ring closure step for total synthesis of (+)-Wortmannin
Wortmannin, a steroid metabolite of the fungi ''Penicillium funiculosum'', '' Talaromyces wortmannii'', is a non-specific, covalent inhibitor of phosphoinositide 3-kinases (PI3Ks). It has an ''in vitro'' inhibitory concentration (''IC''50) of a ...
by Shigehisa, et al. (Figure 2).
Variations and methods
Acetate aldol reactions
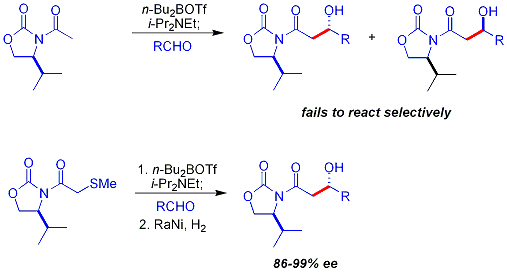
A key limitation to the chiral auxiliary approach described previously is the failure of N-acetylimide
In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applications, ...
s to react selectively. An early approach was to use a temporary thioether group:

Mukaiyama aldol reaction
The Mukaiyama aldol reaction is thenucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions di ...
of silyl enol ethers to aldehydes catalyzed by a Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
such as boron trifluoride
Boron trifluoride is the inorganic compound with the formula BF3. This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
Structure and bondin ...
(as boron trifluoride etherate
Boron trifluoride etherate, strictly boron trifluoride diethyl etherate, or boron trifluoride–ether complex, is the chemical compound with the formula BF3O(C2H5)2, often abbreviated BF3OEt2. It is a colorless liquid, although older samples can a ...
) or titanium tetrachloride. The Mukaiyama aldol reaction does not follow the Zimmerman-Traxler model. Carreira has described particularly useful asymmetric methodology with silyl ketene acetals, noteworthy for its high levels of enantioselectivity and wide substrate scope.
The method works on unbranched aliphatic aldehydes, which are often poor electrophiles for catalytic, asymmetric processes. This may be due to poor electronic and steric differentiation between their enantiofaces.
 The analogous vinylogous Mukaiyama aldol process can also be rendered catalytic and asymmetric. The example shown below works efficiently for aromatic (but not aliphatic) aldehydes and the mechanism is believed to involve a chiral, metal-bound dienolate.
The analogous vinylogous Mukaiyama aldol process can also be rendered catalytic and asymmetric. The example shown below works efficiently for aromatic (but not aliphatic) aldehydes and the mechanism is believed to involve a chiral, metal-bound dienolate.

Crimmins thiazolidinethione aldol
In the Crimmins thiazolidinethione approach a thiazolidinethione performs acetate aldol reactions. and can produce the "Evans syn" or "non-Evans syn" adducts by simply varying the amount of (−)-sparteine. The reaction is believed to proceed via six-membered, titanium-bound transition states, analogous to the proposed transition states for the Evans auxiliary. NOTE: the structure of sparteine shown below is missing a N atom.
Organocatalysis
Chiral secondary amine catalysts catalyze some aldol reactions. These secondary amines form transient enamines when exposed to ketones, which may react enantioselectively with suitable aldehyde electrophiles. The amine reacts with the carbonyl to form an enamine, the enamine acts as an enol-like nucleophile, and then the amine is released from the product all—the amine itself is a catalyst. This enamine catalysis method is a type oforganocatalysis
In organic chemistry, organocatalysis is a form of catalysis in which the rate of a chemical reaction is increased by an organic catalyst. This "organocatalyst" consists of carbon, hydrogen, sulfur and other nonmetal elements found in organic com ...
, since the catalyst is entirely based on a small organic molecule. In a seminal example, proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the prot ...
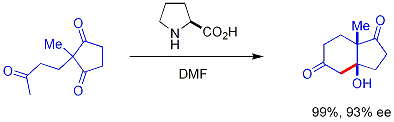
efficiently catalyzed the cyclization of a triketone:
 This reaction is known as the
This reaction is known as the Hajos-Parrish reaction The Hajos–Parrish–Eder–Sauer–Wiechert and Barbas-List reactions in organic chemistry are a family of proline-catalysed asymmetric aldol reactions.
In the 1970s, two research groups discovered (and published) almost simultaneously thei ...
(also known as the Hajos-Parrish-Eder-Sauer-Wiechert reaction, referring to a contemporaneous report from Schering of the reaction under harsher conditions). Under the Hajos-Parrish conditions only a catalytic amount of proline is necessary (3 mol%). There is no danger of an achiral background reaction because the transient enamine intermediates are much more nucleophilic than their parent ketone enols. This strategy offers a simple way of generating enantioselectivity in reactions without using transition metals, which have the possible disadvantages of being toxic or expensive.
Proline-catalyzed aldol reactions do not show any non-linear effects (the enantioselectivity of the products is directly proportional to the enantiopurity of the catalyst). Combined with isotopic labelling evidence and computational studies, the proposed reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ...
for proline-catalyzed aldol reactions is as follows:
 This strategy allows the otherwise challenging cross-aldol reaction between two aldehydes. In general, cross-aldol reactions between aldehydes are typically challenging because they can polymerize easily or react unselectively to give a statistical mixture of products. The first example is shown below:
This strategy allows the otherwise challenging cross-aldol reaction between two aldehydes. In general, cross-aldol reactions between aldehydes are typically challenging because they can polymerize easily or react unselectively to give a statistical mixture of products. The first example is shown below:
 In contrast to the preference for syn adducts typically observed in enolate-based aldol additions, these organocatalyzed aldol additions are anti-selective. In many cases, the organocatalytic conditions are mild enough to avoid polymerization. However, selectivity requires the slow syringe-pump controlled addition of the desired electrophilic partner because both reacting partners typically have enolizable protons. If one aldehyde has no enolizable protons or alpha- or beta-branching, additional control can be achieved.
An elegant demonstration of the power of asymmetric organocatalytic aldol reactions was disclosed by MacMillan and coworkers in 2004 in their synthesis of differentially protected carbohydrates. While traditional synthetic methods accomplish the synthesis of hexoses using variations of iterative protection-deprotection strategies, requiring 8–14 steps, organocatalysis can access many of the same substrates using an efficient two-step protocol involving the proline-catalyzed dimerization of alpha-oxyaldehydes followed by tandem Mukaiyama aldol cyclization.
In contrast to the preference for syn adducts typically observed in enolate-based aldol additions, these organocatalyzed aldol additions are anti-selective. In many cases, the organocatalytic conditions are mild enough to avoid polymerization. However, selectivity requires the slow syringe-pump controlled addition of the desired electrophilic partner because both reacting partners typically have enolizable protons. If one aldehyde has no enolizable protons or alpha- or beta-branching, additional control can be achieved.
An elegant demonstration of the power of asymmetric organocatalytic aldol reactions was disclosed by MacMillan and coworkers in 2004 in their synthesis of differentially protected carbohydrates. While traditional synthetic methods accomplish the synthesis of hexoses using variations of iterative protection-deprotection strategies, requiring 8–14 steps, organocatalysis can access many of the same substrates using an efficient two-step protocol involving the proline-catalyzed dimerization of alpha-oxyaldehydes followed by tandem Mukaiyama aldol cyclization.
 The aldol dimerization of alpha-oxyaldehydes requires that the aldol adduct, itself an aldehyde, be inert to further aldol reactions.
Earlier studies revealed that aldehydes bearing alpha-alkyloxy or alpha-
The aldol dimerization of alpha-oxyaldehydes requires that the aldol adduct, itself an aldehyde, be inert to further aldol reactions.
Earlier studies revealed that aldehydes bearing alpha-alkyloxy or alpha-silyloxy
Silyl ethers are a group of chemical compounds which contain a silicon atom covalent bond, covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used ...
substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as ''side ...
s were suitable for this reaction, while aldehydes bearing Electron-withdrawing group
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications:
*with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of the ...
s such as acetoxy were unreactive. The protected erythrose product could then be converted to four possible sugars via Mukaiyama aldol addition followed by lactol formation. This requires appropriate diastereocontrol in the Mukaiyama aldol addition and the product silyloxycarbenium ion to preferentially cyclize, rather than undergo further aldol reaction. In the end, glucose, mannose, and allose were synthesized:

"Direct" aldol additions
In the usual aldol addition, a carbonyl compound is deprotonated to form the enolate. The enolate is added to an aldehyde or ketone, which forms an alkoxide, which is then protonated on workup. A superior method, in principle, would avoid the requirement for a multistep sequence in favor of a "direct" reaction that could be done in a single process step. One idea is to generate the enolate using a metal catalyst that is released after the aldol addition mechanism. The general problem is that the addition generates an alkoxide, which is much more basic than the starting materials. This product binds tightly to the metal, preventing it from reacting with additional carbonyl reactants. One approach, demonstrated by Evans, is to silylate the aldol adduct. A silicon reagent such as TMSCl is added in the reaction, which replaces the metal on the alkoxide, allowing
One approach, demonstrated by Evans, is to silylate the aldol adduct. A silicon reagent such as TMSCl is added in the reaction, which replaces the metal on the alkoxide, allowing turnover
Turnover or turn over may refer to:
Arts, entertainment, and media
*''Turn Over'', a 1988 live album by Japanese band Show-Ya
* Turnover (band), an American rock band
*"Turnover", a song on Fugazi's 1990 album '' Repeater''
*''Turnover'', a Japane ...
of the metal catalyst. Minimizing the number of reaction steps and amount of reactive chemicals used leads to a cost-effective and industrially useful reaction.

Biological aldol reactions
Examples of aldol reactions in biochemistry include the splitting offructose-1,6-bisphosphate
Fructose 1,6-bisphosphate, also known as Harden-Young ester, is fructose sugar phosphorylated on carbons 1 and 6 (i.e., is a fructosephosphate). The β-D-form of this compound is common in cells. Upon entering the cell, most glucose and fructos ...
into dihydroxyacetone and glyceraldehyde-3-phosphate in the fourth stage of glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvate (). The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH ...
, which is an example of a reverse ("retro") aldol reaction catalyzed by the enzyme aldolase A
Aldolase A (ALDOA, or ALDA), also known as fructose-bisphosphate aldolase, is an enzyme that in humans is encoded by the ''ALDOA'' gene on chromosome 16.
The protein encoded by this gene is a glycolytic enzyme that catalyzes the reversible conv ...
(also known as fructose-1,6-bisphosphate aldolase).
In the glyoxylate cycle of plants and some prokaryotes, isocitrate lyase
Isocitrate lyase (), or ICL, is an enzyme in the glyoxylate cycle that catalyzes the cleavage of isocitrate to succinate and glyoxylate. Together with malate synthase, it bypasses the two decarboxylation steps of the tricarboxylic acid cycle (TC ...
produces glyoxylate and succinate from isocitrate
Isocitric acid is a structural isomer of citric acid. Since citric acid and isocitric acid are structural isomers, they share similar physical and chemical properties. Due to these similar properties, it is difficult to separate the isomers. Salt ...
. Following deprotonation of the OH group, isocitrate lyase cleaves isocitrate into the four-carbon succinate and the two-carbon glyoxylate by an aldol cleavage reaction. This cleavage is similar mechanistically to the aldolase A reaction of glycolysis.
See also
*Aldol–Tishchenko reaction The Aldol–Tishchenko reaction is a tandem reaction involving an aldol reaction and a Tishchenko reaction. In organic synthesis it is a method to convert aldehydes and ketones into 1,3-hydroxyl compounds. The reaction sequence in many examples star ...
* Baylis–Hillman reaction
* Ivanov reaction
The Ivanov reaction is the chemical reaction of the dianions (endiolates) of aryl acetic acids (Ivanov reagents) with electrophiles, primarily carbonyl compounds or isocyanates. The reaction was named after the Bulgarian organic chemist, Academicia ...
* Reformatsky reaction
References
External links
* Chem 206, 215 Lecture Notes (2003, 2006) by D. A. Evans, A. G. Myers, ''et al.'', Harvard University (pp. 345, 936) {{Organic reactions Addition reactions Carbon-carbon bond forming reactions