Ynone on:
[Wikipedia]
[Google]
[Amazon]
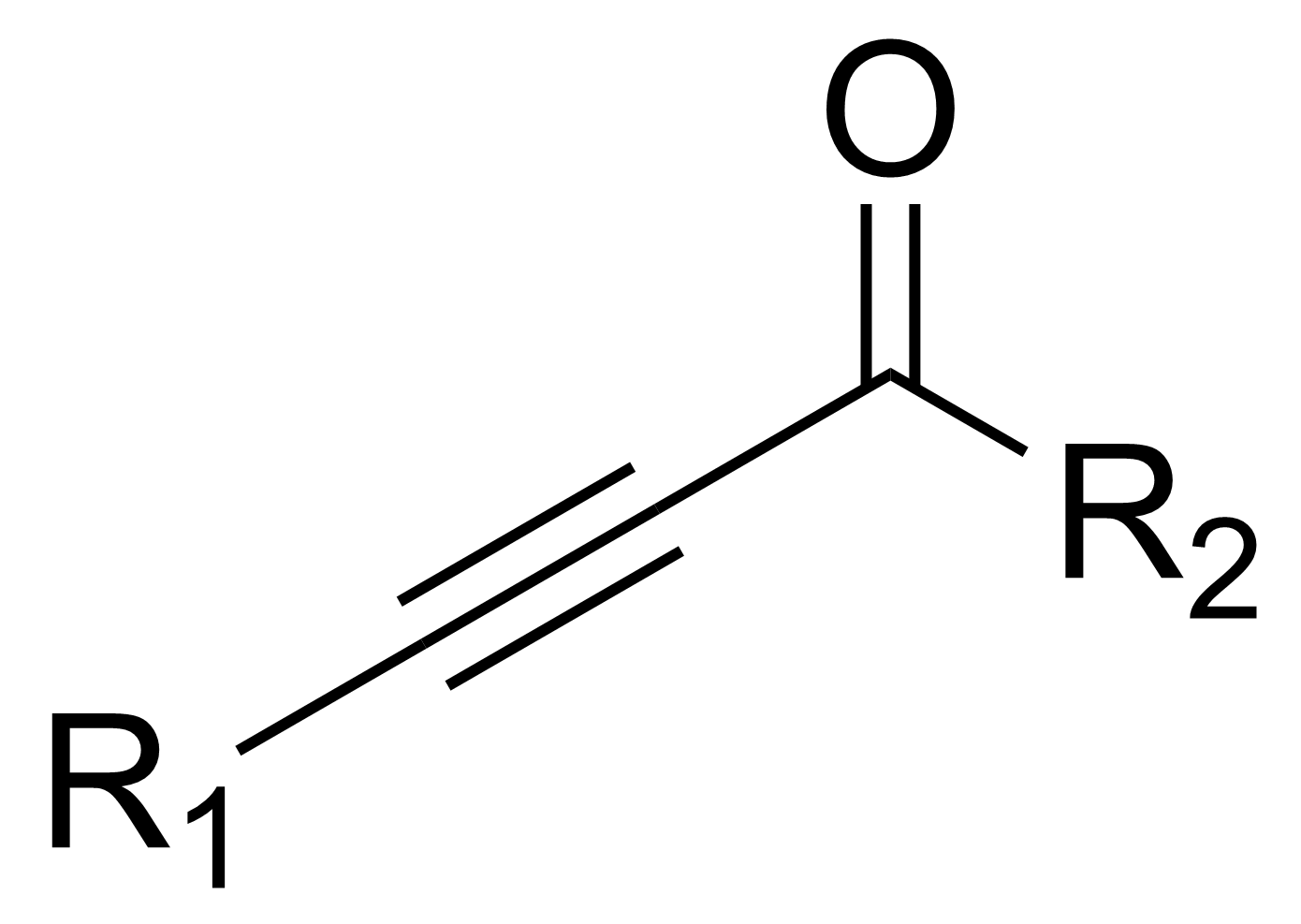 In
In
 An alternative is the direct coupling of an
An alternative is the direct coupling of an  Other methods utilize an oxidative cleavage of an
Other methods utilize an oxidative cleavage of an
 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
, an ynone is an organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ...
containing a ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
() functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
and a triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order o ...
. Many ynones are α,β-ynones, where the carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
and alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
groups are conjugated. Capillin
Capillin is a naturally occurring organic compound with the chemical formula . The structure contains acetophenone and a polyyne (pentadiynyl) portion, conjugated together as an ynone.
Chemical taxonomy
Capillin is found in the essential oil of a ...
is a naturally occurring example. Some ynones are not conjugated.
Synthesis of α,β-ynones
One method for synthesizing ynones is the acyl substitution reaction of an alkynyldimethylaluminum with anacyl chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
. An alkynyldimethylaluminum compound is the reaction product of trimethylaluminum
Trimethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2( CH3)6 (abbreviated as Al2Me6 or TMA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industriall ...
and a terminal alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
.
 An alternative is the direct coupling of an
An alternative is the direct coupling of an acyl chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
with a terminal alkyne, using a copper-based nanocatalyst:
 Other methods utilize an oxidative cleavage of an
Other methods utilize an oxidative cleavage of an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
, followed by reaction with a hypervalent alkynyl iodide, using a gold catalyst.
An alternative but longer synthetic method involves the reaction of an alkynyllithium compound with an aldehyde. The reaction produces a secondary alcohol that then can be oxidized via the Swern oxidation
The Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide (DMSO) and an organic base, such as triethylamine. It is one ...
.
Synthesis of β,γ- and γ,δ-ynones
Terminal alkynes add across α,β-unsaturated ketones in the presence of palladium catalysts. The reaction affords γ,δ-ynones. Terminal alkynes add across epoxides to given yneols, which can be oxidized to give β,γ-ynones.Further reading
*Bis-ynones can undergo an intramolecularcycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
to form furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.
Furan is a colorless, flammable, highly ...
derivatives.
See also
*Diketone
In organic chemistry, a dicarbonyl is a molecule containing two carbonyl () groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls ...
References
External links
* {{Functional group Functional groups Ketones Alkyne derivatives