Workup (chemistry) on:
[Wikipedia]
[Google]
[Amazon]
In chemistry, work-up refers to the series of manipulations required to isolate and purify the product(s) of a 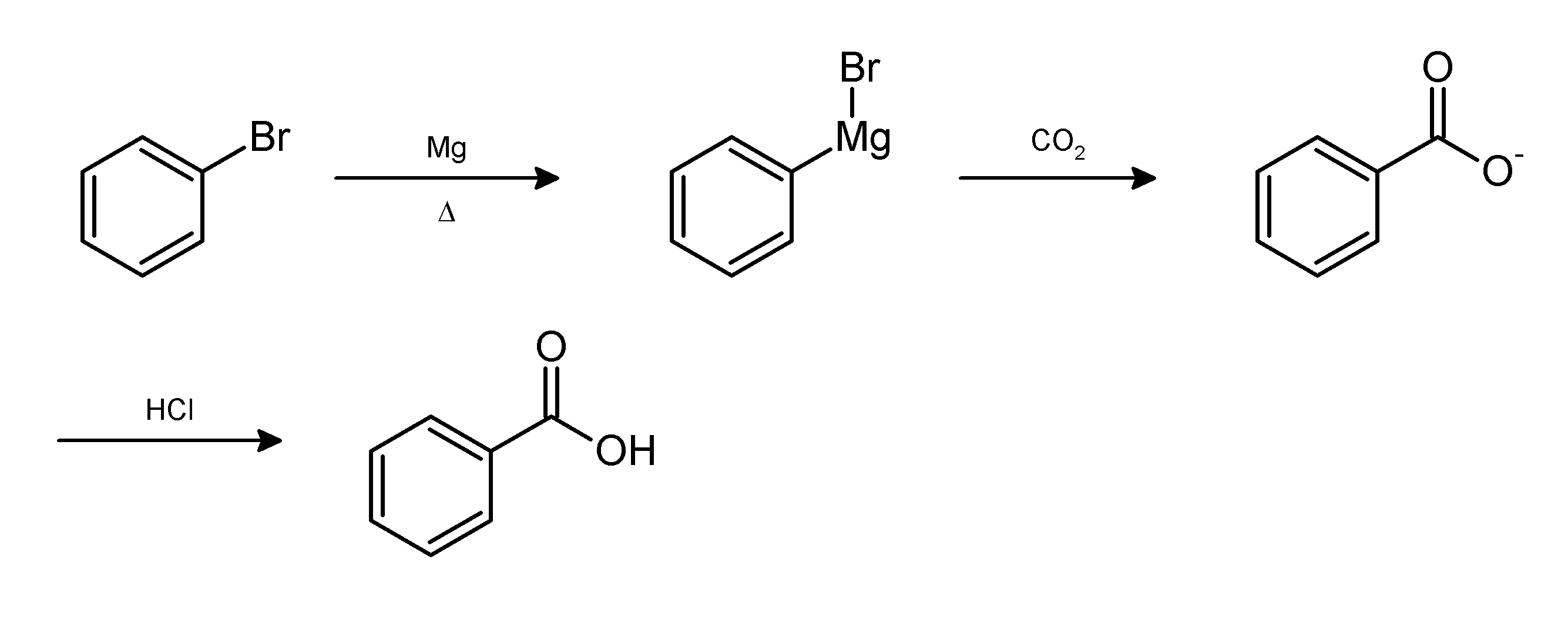 *The reaction mixture containing the Grignard reagent is allowed to warm to room temperature in a water bath to allow excess dry ice to evaporate
*Any remaining Grignard reagent is quenched by the addition of water
*Dilute
*The reaction mixture containing the Grignard reagent is allowed to warm to room temperature in a water bath to allow excess dry ice to evaporate
*Any remaining Grignard reagent is quenched by the addition of water
*Dilute
chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
.
Typically, these manipulations may include:
* quenching a reaction to deactivate any unreacted reagents.
* cooling the reaction mixture or adding an ''antisolvent
Salting out (also known as salt-induced precipitation, salt fractionation, anti-solvent crystallization, precipitation crystallization, or drowning out) is a purification technique that utilizes the reduced solubility of certain molecules in a s ...
'' to induce precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls under gravitational pull from clouds. The main forms of precipitation include drizzle, rain, sleet, snow, ice pellets, graupel and hail. ...
, and collecting or removing the solids by filtration, decantation
Decantation is a process for the separation of mixtures of immiscible liquids or of a liquid and a solid mixture such as a suspension. The layer closer to the top of the container—the less dense of the two liquids, or the liquid from which t ...
, or centrifugation
* removal of solvents by evaporation
* separating the reaction mixture into organic and aqueous layers by liquid-liquid extraction
* purification by chromatography
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system ( ...
, distillation
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the heat ...
or recrystallization
For example, the Grignard reaction
The Grignard reaction () is an organometallic chemical reaction in which alkyl, allyl, vinyl, or aryl-magnesium halides (Grignard reagent) is added to a carbonyl group in an aldehyde or ketone. This reaction is important for the formation of ...
between phenylmagnesium bromide and carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
in the form of dry ice
Dry ice is the solid form of carbon dioxide. It is commonly used for temporary refrigeration as CO2 does not have a liquid state at normal atmospheric pressure and sublimates directly from the solid state to the gas state. It is used primarily ...
gives the conjugate base of benzoic acid
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, ...
. The desired product, benzoic acid, is obtained by the following work-up:{{cite book , title = Introduction to Organic Laboratory Techniques: A Small Scale Approach , author = Donald L. Pavia , year = 2004 , publisher = Thomson Brooks/Cole , isbn = 0-534-40833-8 , pages = 312–314
 *The reaction mixture containing the Grignard reagent is allowed to warm to room temperature in a water bath to allow excess dry ice to evaporate
*Any remaining Grignard reagent is quenched by the addition of water
*Dilute
*The reaction mixture containing the Grignard reagent is allowed to warm to room temperature in a water bath to allow excess dry ice to evaporate
*Any remaining Grignard reagent is quenched by the addition of water
*Dilute hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
is added to the reaction mixture to protonate the benzoate salts, as well as to dissolve the magnesium salts. White solids of impure benzoic acid are obtained.
*The benzoic acid is decanted to remove the aqueous solution of impurities, more water is added, and the mixture is brought to a boil with more water added to give a homogeneous solution.
*The solution is allowed to cool slowly to room temperature, then in an ice bath to recrystallize benzoic acid.
*The recrystallized benzoic acid crystals are collected on a Buchner funnel and are allowed to air-dry to give pure benzoic acid.
See also
*Flow chemistry
In flow chemistry, a chemical reaction is run in a continuously flowing stream rather than in batch production. In other words, pumps move fluid into a reactor, and where tubes join one another, the fluids contact one another. If these fluids ar ...
References